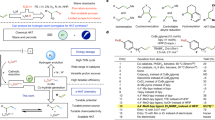
Abstract
In recently years, the field of organic chemistry has seen a growing interest in the development of environmentally friendly and efficient synthetic methods. In this context, we introduce a new electro-organic approach for the synthesis of methyl cinnamate derivatives through the Heck reaction, carried out under green conditions. The conventional Heck reaction, widely used for synthesizing diverse compounds, suffers from drawbacks like the use of toxic solvents, harsh reaction conditions, and the generation of waste. To address these challenges, we employed an electrochemical method, offering a more sustainable alternative. Our electro-organic process utilized a two-electrode setup with easily available anode and cathode materials. Through the application of an appropriate potential difference, both the aryl halide and olefin substrates were electrochemically activated, leading to the formation of the desired methyl cinnamate derivatives. This innovative approach offers several significant advantages. Firstly, it eliminates the need for toxic catalysts, reducing the environmental impact related to waste disposal. Secondly, the mild reaction conditions allow for the use of a broad range of functional groups, enabling the synthesis of diverse methyl cinnamate derivatives. Moreover, the electrochemical approach demonstrates exceptional selectivity and efficiency, resulting in high product yields. Additionally, the method is easily scalable, making it suitable for large-scale production. The affordability and accessibility of the electrode materials further contribute to the sustainability of the process. In summary, our electro-organic method represents a greener and more efficient strategy for synthesizing methyl cinnamate derivatives via the Heck reaction. It not only addresses the limitations of conventional methods but also aligns with the principles of sustainable chemistry. We expect this novel methodology to find widespread applications in the synthesis of various important compounds, promoting the development of more sustainable chemical processes.
Graphical Abstract






Similar content being viewed by others
References
Jagtap S (2017) Catalysts 7:267
Shibasaki M, Vogl EM, Ohshima T (2004) Adv Synth Catal 346:1533–1552
Link JT (2004) Org React 60:157–561
Zou Y, Zhou JS (2014) Chem Commun 50:3725–3728
Wu X-F, Neumann H, Spannenberg A, Schulz T, Jiao H, Beller M (2010) J Am Chem Soc 132:14596–14602
Trzeciak AM, Ziółkowski JJ (2005) Coord Chem Rev 249:2308–2322
Prashad M (2004) Organometall Process Chem. 4:181–203
Burello E, Rothenberg G (2003) Adv Synth Catal 345:1334–1340
D Paul S Das S Saha H Sharma RK Goswami 2021 Eur J Org Chem 2021 2057 2076
Dounay AB, Overman LE (2003) Chem Rev 103:2945–2964
Ghosh T (2019) ChemistrySelect 4:4747–4755
Devendar P, Qu R-Y, Kang W-M, He B, Yang G-F (2018) J Agric Food Chem 66:8914–8934
Y. Zhu, W. Dong and W. Tang, Adv. Agrochem.
Torborg C, Beller M (2009) Adv Synth Catal 351:3027–3043
M. OESTREICH, .
Crisp GT (1998) Chem Soc Rev 27:427–436
De Vries JG (2001) Can J Chem 79:1086–1092
Kong K, Enquist JA Jr, McCallum ME, Smith GM, Matsumaru T, Menhaji-Klotz E, Wood JL (2013) J Am Chem Soc 135:10890–10893
Biffis A, Centomo P, Del Zotto A, Zecca M (2018) Chem Rev 118:2249–2295
Madasu SB, Vekariya NA, Kiran MH, Gupta B, Islam A, Douglas PS, Babu KR (2012) Beilstein J Org Chem 8:1400–1405
Nakano S, Hamada Y, Nemoto T (2018) Tetrahedron Lett 59:760–762
Monaco A, Szulc BR, Rao ZX, Barniol-Xicota M, Sehailia M, Borges BMA, Hilton ST (2017) Chem Eur J 23:4750–4755
Park KHK, Chen DY-K (2018) Chem Commun 54:13018–13021
Lu Z, Yang M, Chen P, Xiong X, Li A (2014) Angew Chemie 126:14060–14064
Choi J, Kim H, Park S, Tae J (2013) Synlett 24:379–382
Xu L, Wang C, Gao Z, Zhao Y-M (2018) J Am Chem Soc 140:5653–5658
Fu C, Zhang Y, Xuan J, Zhu C, Wang B, Ding H (2014) Org Lett 16:3376–3379
Mizoroki T, Mori K, Ozaki A (1971) Bull Chem Soc Jpn 44:581
Heck RF, Nolley JP Jr (1972) J Org Chem 37:2320–2322
Gutmann B, Glasnov TN, Razzaq T, Goessler W, Roberge DM, Kappe CO (2011) Beilstein J Org Chem 7:503–517
Jiao K-J, Xing Y-K, Yang Q-L, Qiu H, Mei T-S (2020) Acc Chem Res 53:300–310
Kärkäs MD (2018) Chem Soc Rev 47:5786–5865
Mohammadi AA, Makarem S, Ahdenov R, Notash NA (2020) Mol Divers 24:763–770
Raya I, Altimari US, Alami BG, Srikanth S, Gatea MA, Romero-Parra RM, Barboza-Arenas LA, Mustafa YF (2023) J Mol Struct. 5:136271
Laudadio G, De Smet W, Struik L, Cao Y, Noël T (2018) J Flow Chem 8:157–165
Watts K, Baker A, Wirth T (2015) J Flow Chem 4:2–11
O’Brien AG, Maruyama A, Inokuma Y, Fujita M, Baran PS, Blackmond DG (2014) Angew Chemie Int Ed 53:11868–11871
Palasz PD, Utley JHP, Hardstone JD (1984) J Chem Soc Perkin Trans 2:807–813
Ucheniya K, Chouhan A, Yadav L, Jat PK, Badsara SS (2023) J Org Chem 88:6096–6107
Pollok D, Waldvogel SR (2020) Chem Sci 11:12386–12400
Biddinger EJ, Modestino MA (2020) Electrochem Soc Interface 29:43
Ahdenov R, Mohammadi AA, Makarem S, Taheri S, Mollabagher H (2022) Heterocycl Commun 28:67–74
Valentini F, Ferlin F, Lilli S, Marrocchi A, Ping L, Gu Y, Vaccaro L (2021) Green Chem 23:5887–5895
Hochberger-Roa F, Cortés-Mendoza S, Gallardo-Rosas D, Toscano RA, Ortega-Alfaro MC, López-Cortés JG (2019) Adv Synth Catal 361:4055–4064
Guzman JD, Mortazavi PN, Munshi T, Evangelopoulos D, McHugh TD, Gibbons S, Malkinson J, Bhakta S (2014) Medchemcomm 5:47–50
Ojha S, Panda N (2022) Org Biomol Chem 20:1292–1298
Kochurin MA, Ismagilova AR, Zakusilo DN, Khoroshilova OV, Boyarskaya IA, Vasilyev AV (2022) New J Chem 46:12041–12053
Chitsomkhuan S, Buakaew S, Samec JSM, Chuawong P, Kuntiyong P (2022) Synlett 33:1353–1356
Yoshioka E, Takahashi H, Kubo A, Ohno M, Watanabe F, Shiono R, Miyazaki Y, Miyabe H (2022) Synthesis (Stuttg) 54:5520–5528
Kumar A, Tateyama S, Yasaki K, Ali MA, Takaya N, Singh R, Kaneko T (2016) Polymer (Guildf) 83:182–189
Yoshioka E, Inoue M, Nagoshi Y, Kobayashi A, Mizobuchi R, Kawashima A, Kohtani S, Miyabe H (2018) J Org Chem 83:8962–8970
Mahmoudi H, Valentini F, Ferlin F, Bivona LA, Anastasiou I, Fusaro L, Aprile C, Marrocchi A, Vaccaro L (2019) Green Chem 21:355–360
Mamidala R, Mukundam V, Dhanunjayarao K, Venkatasubbaiah K (2015) Dalt Trans 44:5805–5809
Brizzi G, Puzzarini C, Perrin A, Orphal J, Willner H, Garcia P (2005) J Mol Struct 742:37–41
Etzkorn FA, Ferguson JL (2023) Angew Chemie Int Ed 62:e202209768
Martínez J, Cortés JF, Miranda R (2022) Processes 10:1274
Horváth IT, Anastas PT (2007) Chem Rev 107:2167–2168
Acknowledgements
This study is supported via funding from Prince Sattan bin Abdulaziz University project number (PSAU/2023/R/1444)
Author information
Authors and Affiliations
Contributions
RHA, SISA, SSA write main manuscript SSA, AHA prepared figures, AH prepared tables. Writing - Original manuscript Draft, Review, and Editing: MKA. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Althomali, R.H., Al-Hawary, S.I.S., Abdullaev, S.S. et al. A New and Efficient Electro Organic Method for Synthesis of Methyl Cinnamate Derivatives via Heck Reaction Under Green Conditions. Catal Surv Asia 28, 200–208 (2024). https://doi.org/10.1007/s10563-023-09418-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10563-023-09418-7