Abstract
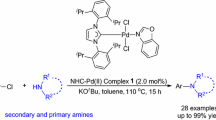
Six novel palladium N-heterocyclic carbene complexes bearing different ancillary ligands such as pyridine, 1-methylimidazole, 4,5-dimethylthiazole and 3-bromoquinoline have been successfully prepared in a one-step process. These complexes were used as pre-catalysts in the Buchwald–Hartwig amination of cyclic secondary amines with aryl chlorides. A range of cyclic secondary amines were tolerated to a wide variety of aryl chlorides in presence of 1 mol% catalyst loading under mild conditions. Under the tested reaction conditions, the expected N-arylamines were obtained in moderate to high yields. The results showed that these novel palladium complexes are effective catalysts for the Buchwald–Hartwig amination.
Graphic Abstract



Similar content being viewed by others
References
Lawrence SA (ed) (2004) Amines: synthesis properties and applications. Cambridge University, Cambridge
Brown BR (ed) (2004) The organic chemistry of aliphatic nitrogen compounds. Cambridge University, Cambridge
Weissermel K, Arpe HJ (eds) (1997) Industrial organic chemistry. Wiley-VCH, Weinheim
Belfield A, Brown GR, Foubister AJ (1999) Tetrahedron 55:11399–11428
Goodbrand HB, Hu N-X (1999) J Org Chem 64:670–674
O’Hagan D (2000) Nat Prod Rep 17:435–446
Kazemi M, Mohammadi M (2020) Appl Organometal Chem 34:e5400
Ghorbani-Choghamarani A, Mohammadi M, Tamoradi T, Ghadermazi M (2019) Polyhedron 158:25–35
Guram AS, Rennels RA, Buchwald SL (1995) Angew Chem 107:1456–1459; Angew Chem Int Ed Engl 34:1348–1350
Louie J, Hartwig JF (1995) Tetrahedron Lett 36:3609–3612
Beller M, Bolm C (1998) Transition metals for organic synthesis: building blocks and fine chemicals. Wiley-VCH, Weinheim
Tamao K, Miyaura N (2002) Introduction to cross‐coupling reactions. In: Miyaura N (ed) Cross‐coupling reactions: a practical guide. Springer, Berlin
Rama Suresh R, Kumara Swamy KC (2009) Tetrahedron Lett 50:6004–6007
Lundgren RJ, Stradiotto M (2012) Chem Eur J 18:9758–9769
Bariwal J, Van der Eycken E (2013) Chem Soc Rev 42:9283–9303
Ruiz-Castillo P, Buchwald SL (2016) Chem Rev 116:12564–12649
Lan X-B, Li Y-W, Li Y-F, Shen D-S, Ke Z-F, Liu F-S (2017) J Org Chem 82:2914–2925
Dorel R, Grugel CP, Haydl AM (2019) Angew Chem Int Ed 58:17118–17129
Cao Q, Nicholson WI, Jones AC, Browne DL (2019) Org Biomol Chem 17:1722–1726
Beletskaya IP, Averin AD (2021) Russ Chem Rev 90:1359–1396
Hartwig JF (2003) Palladium-catalyzed amination of aryl halides and related reactions. In: Handbook of organopalladium chemistry for organic synthesis, Chapter III.3.2. Wiley-Interscience, New York
Wolfe JP, Wagaw S, Buchwald SL (1996) J Am Chem Soc 118:7215–7216
Driver M, Hartwig J (1996) J Am Chem Soc 118:7217–7218
Ackermann L, Spatz JH, Gschrei CJ, Born R, Althammer A (2006) Angew Chem Int Ed 45:7627–7630
Hartwig JF (2008) Acc Chem Res 41:1534–1544
Wong SM, Yuen OY, Choy PY, Kwong FY (2015) Coord Chem Rev 158:293–294
Heravi MM, Kheilkordi Z, Zadsirjan V, Haydari M, Malmir M (2018) J Organomet Chem 861:17–104
Nolan SP (ed) (2006) N-Heterocyclic Carbenes in Synthesis. Wiley, Weinheim
Glorius F (ed) (2007) Topics in organometallic chemistry: n-heterocyclic carbenes in transition metal catalysis, vol 21. Springer, Heidelberg
Nolan SP (2014) N-Heterocyclic carbenes: effective tools for organometallic synthesis. Wiley-VCH, Weinheim
Huynh HV (2017) The organometallic chemistry of N-heterocyclic carbenes. Wiley, Hoboken
Peris E (2017) Chem Rev 118:9988–10031
Dharani S, Kalaiarasi G, Lynch VM, Prabhakaran R (2023) Appl Organometal Chem 37:e7076
Yang J (2017) Appl Organometal Chem 31:e3734
Kaloğlu M, Gürbüz N, Yıldırım İ, Özdemir N, Özdemir İ (2020) Appl Organometal Chem 34:e5387
Özdemir İ, Demir Düşünceli S, Kaloğlu N, Achard M, Bruneau C (2015) J Organomet Chem 799–800:311–315
Kaloğlu M, Kaloğlu N, Özdemir İ, Günal S, Özdemir İ (2016) Bioorg Med Chem 24:3649–3656
Kaloğlu M, Kaloğlu N, Özdemir İ (2018) Chin J Chem 36:837–844
Kaloğlu N (2019) Tetrahedron 75:2265–2272
Kaloğlu M, Kaloğlu N, Yıldırım İ, Özdemir N, Özdemir İ (2020) J Mol Struc 1206:127668
Kaloğlu M, Kaloğlu N, Özdemir İ (2021) Catal Lett 151:3197–3212
Kaloğlu M, Kaloğlu N, Özdemir N, Özdemir İ (2021) Res Chem Intermed 47:2821–2843
Özdemir N, Kaloğlu M, Kaloğlu N, Gürbüz N, Özdemir İ (2021) Inorg Nano-Met Chem 52:493–504
Marion N, Navarro O, Mei J, Stevens ED, Scott NM, Nolan SP (2006) J Am Chem Soc 128:4101–4111
Organ MG, Abdel-Hadi M, Avola S, Dubovyk I, Hadei N, Kantchev EAB, O’Brien CJ, Sayah M, Valente C (2008) Chem Eur J 14:2443–2452
Özdemir İ, Demir S, Şahin O, Büyükgüngör O, Çetinkaya B (2010) J Organomet Chem 695:1555–1560
Fortman GC, Nolan SP (2011) Chem Soc Rev 40:5151–5169
Hoi KH, Çalimsiz S, Froese RDJ, Hopkinson AC, Organ MG (2011) Chem Eur J 17:3086–3090
Demir S, Özdemir İ, Çetinkaya B, Arslan H, VanDerveer D (2011) Polyhedron 30:195–200
Fang W, Jiang J, Xu Y, Zhou J, Tu T (2013) Tetrahedron 69:673–679
Froese RDJ, Lombardi C, Pompeo M, Rucker RP, Organ MG (2017) Acc Chem Res 50:2244–2253
Huang F-D, Xu C, Lu D-D, Shen D-S, Li T, Liu F-S (2018) J Org Chem 83:9144–9155
Reddy MVK, Anusha G, Reddy PVG (2020) New J Chem 27:11694–11703
Chen M-T, Vicic DA, Chain WJ, Turner ML, Navarro O (2011) Organometallics 30:6770–6773
O’Brien CJ, Kantchev EAB, Valente C, Hadei N, Chass GA, Lough A, Hopkinson AC, Organ MG (2006) Chem Eur J 12:4743–4748
Zhu L, Gao T-T, Shao L-X (2011) Tetrahedron 67:5150–5155
Chen W-X, Shao L-X (2012) J Org Chem 77:9236–9239
Zhu L, Ye Y-M, Shao L-X (2012) Tetrahedron 68:2414–2420
Zhang Z-M, Xu Y-T, Shao L-X (2021) J Organomet Chem 940:121683
Sun K-X, Zhou J-H, He Q-W, Shao L-X, Lu J-M (2020) Tetrahedron 76:130944
Chen MT, Vicic DA, Turner ML, Navarro O (2011) Organometallics 30:5052–5056
Huang P, Wang Y-X, Yu H-F, Lu J-M (2014) Organometallics 33:1587–1593
Liu F, Zhu Y-R, Song L-G, Lu J-M (2016) Org Biomol Chem 14:2563–2571
Zhang Z-M, Gao Y-J, Lu J-M (2017) Tetrahedron 73:7308–7314
Liu F, Hu Y-Y, Li D, Zhou Q, Lu J-M (2018) Tetrahedron 74:5683–5690
Karataş MO, Özdemir N, Alıcı B, Özdemir İ (2020) Polyhedron 176:114271
Kaloğlu M, Özdemir İ, Dorcet V, Bruneau C, Doucet H (2017) Eur J Inorg Chem 10:1382–1391
Kaloğlu M, Slimani I, Özdemir N, Gürbüz N, Hamdi N, Özdemir İ (2021) J Organomet Chem 951:122013
Guo D-L, Huang H, Xu J-Y, Jiang H-L, Liu H (2008) Org Lett 10:4513–4516
Sunesson Y, Limé E, Nilsson Lill SO, Meadows RE, Norrby P-O (2014) J Org Chem 79:11961–11969
Meyers C, Maes BUW, Loones KTJ, Bal G, Lemière GLF, Dommisse RA (2004) J Org Chem 69:6010–6017
Christensen H, Kiil S, Dam-Johansen K (2006) Org Process Res Dev 10:762–769
Esposito O, Gois PMP, de K. Lewis AK, Caddick S, Cloke FGN, Hitchcock PB (2008) Organometallics 27:6411–6418
Zhao X-Y, Zhou Q, Lu J-M (2016) RSC Adv 6:24484–24490
Barker TJ, Jarvo ER (2009) J Am Chem Soc 131:15598–15599
Hatakeyama T, Yoshimoto Y, Ghorai SK, Nakamura M (2010) Org Lett 12:1516–1519
Qian X, Yu Z-L, Auffrant A, Gosmini C (2013) Chem Eur J 19:6225–6229
Manolikakes G, Gavryushin A, Knochel P (2008) J Org Chem 73:1429–1434
Gao C-Y, Yang L-M (2008) J Org Chem 73:1624–1627
Lipshutz BH, Frieman BA, Lee CT, Lower S, Nihan DM, Taft BR (2006) Chem Asian J 1:417–429
Karataş MO (2019) J Organomet Chem 899:120906
Acknowledgements
Support of İnönü University Research Fund (İ.Ü. B.A.P. Project No: FBA-2022-2844) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaloğlu, M., Özdemir, İ. Palladium N-Heterocyclic Carbene Pre-catalysts Bearing Different Ancillary Ligands for the Buchwald–Hartwig Amination of Cyclic Secondary Amines with Aryl Chlorides. Catal Lett 154, 1479–1494 (2024). https://doi.org/10.1007/s10562-023-04432-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-023-04432-w