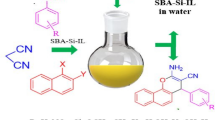
Abstract
A novel nano-Fe3O4 supported Bronsted acidic ionic liquid ([nano-Fe3O4@BenzImi]SO3H) has been prepared by multi-step procedure and characterized by various analytical techniques. The [nano-Fe3O4@BenzImi]SO3H has been employed as a heterogeneous catalyst in the multi-component synthesis of dihydro-1H-pyrazolylnaphthalene-1,4-diones in high yields using ethanol as a green solvent. The [nano-Fe3O4@BenzImi]SO3H could be recycled up to seven catalytic cycles without a significant decrease in the yield of the products.
Graphical Abstract














Similar content being viewed by others
References
Davis JH Jr (2004) Task-specific ionic liquids. Chem Lett 33:1072–1077. https://doi.org/10.1246/CL.2004.1072
Yue C, Fang D, Liu L, Yi TF (2011) Synthesis and application of task-specific ionic liquids used as catalysts and/or solvents in organic unit reactions. J Mol Liq 163:99–121. https://doi.org/10.1016/J.MOLLIQ.2011.09.001
Padvi SA, Dalal DS (2020) Task-specific ionic liquids as a green catalysts and solvents for organic synthesis. Curr Green Chem 7:105–119. https://doi.org/10.2174/2213346107666200115153051
Cole AC, Jensen JL, Ntai I et al (2002) Novel Brønsted acidic ionic liquids and their use as dual solvent−catalysts. J Am Chem Soc 124:5962–5963. https://doi.org/10.1021/JA026290W
Song H, Li Z, Chen J, Xia C (2011) Brönsted acidic ionic liquids as efficient and recyclable catalysts for the carbonylation of formaldehyde. Catal Lett 142(1):81–86. https://doi.org/10.1007/S10562-011-0708-X
Vafaeezadeh M, Alinezhad H (2016) Brønsted acidic ionic liquids: green catalysts for essential organic reactions. J Mol Liq 218:95–105. https://doi.org/10.1016/J.MOLLIQ.2016.02.017
Srivastava R (2010) Assessment of the catalytic activities of novel Brönsted acidic ionic liquid catalysts. Catal Lett 139(1):17–25. https://doi.org/10.1007/S10562-010-0404-2
Nouri Sefat M, Saberi D, Niknam K (2011) Preparation of silica-based ionic liquid an efficient and recyclable catalyst for one-pot synthesis of α-aminonitriles. Catal Lett 141(11):1713–1720. https://doi.org/10.1007/S10562-011-0696-X
Ratti R (2014) Ionic liquids: synthesis and applications in catalysis. Adv Chem 2014:1–16. https://doi.org/10.1155/2014/729842
Song D, Liu J, Zhang C, Guo Y (2021) Design of Brønsted acidic ionic liquid functionalized mesoporous organosilica nanospheres for efficient synthesis of ethyl levulinate and levulinic acid from 5-hydroxymethylfurfural. Catal Sci Technol 11:1827–1842. https://doi.org/10.1039/D0CY01941K
Zhang Q, Luo J, Wei Y (2010) A silica gel supported dual acidic ionic liquid: an efficient and recyclable heterogeneous catalyst for the one-pot synthesis of amidoalkyl naphthols. Green Chem 12:2246–2254. https://doi.org/10.1039/C0GC00472C
Kotadia DA, Soni SS (2013) Sulfonic acid functionalized solid acid: an alternative eco-friendly approach for transesterification of non-edible oils with high free fatty acids. Monatsh Chem 144(11):1735–1741. https://doi.org/10.1007/S00706-013-1041-4
Bian Y, Shan Q, Guo C et al (2021) Biodiesel production over esterification catalyzed by a novel poly(acidic ionic liquid)s. Catal Lett 151(12):3523–3531. https://doi.org/10.1007/S10562-021-03592-X
Bhongale PV, Joshi SS, Mali NA (2022) Reusable and efficient polystyrene immobilized ionic liquid catalyst for batch and flow methylation of hydroquinone. Catal Lett. https://doi.org/10.1007/S10562-022-03918-3
Amarasekara AS, Owereh OS (2010) Synthesis of a sulfonic acid functionalized acidic ionic liquid modified silica catalyst and applications in the hydrolysis of cellulose. Catal Commun 11:1072–1075. https://doi.org/10.1016/J.CATCOM.2010.05.012
Kotadia DA, Soni SS (2012) Silica gel supported –SO3H functionalised benzimidazolium based ionic liquid as a mild and effective catalyst for rapid synthesis of 1-amidoalkyl naphthols. J Mol Catal A 353–354:44–49. https://doi.org/10.1016/J.MOLCATA.2011.11.003
Safaei S, Mohammadpoor-Baltork I, Khosropour AR et al (2013) Nano-silica supported acidic ionic liquid as an efficient catalyst for the multi-component synthesis of indazolophthalazine-triones and bis-indazolophthalazine-triones. Catal Sci Technol 3:2717–2722. https://doi.org/10.1039/C3CY00344B
Skoda-Földes R (2014) The use of supported acidic ionic liquids in organic synthesis. Molecules 19:8840–8884. https://doi.org/10.3390/MOLECULES19078840
Gajare S, Patil A, Hangirgekar S et al (2020) Green synthesis of quinolines via A3-coupling by using graphene oxide-supported Brønsted acidic ionic liquid. Res Chem Intermed 46(5):2417–2436. https://doi.org/10.1007/S11164-020-04099-7
Centi G, Perathoner S (2003) Catalysis and sustainable (green) chemistry. Catal Today 77:287–297. https://doi.org/10.1016/S0920-5861(02)00374-7
Govan J, Gun’ko YK (2014) Recent advances in the application of magnetic nanoparticles as a support for homogeneous catalysts. Nanomaterials 4:222–241. https://doi.org/10.3390/NANO4020222
Abu-Dief AM, Abdel-Fatah SM (2018) Development and functionalization of magnetic nanoparticles as powerful and green catalysts for organic synthesis. Beni-Suef Univ J Basic Appl Sci 7:55–67. https://doi.org/10.1016/J.BJBAS.2017.05.008
Hasany S, Abdurahman N, Sunarti A, Jose R (2013) Magnetic iron oxide nanoparticles: chemical synthesis and applications review. Curr Nanosci 9:561–575. https://doi.org/10.2174/15734137113099990085
Ghazanfari MR, Kashefi M, Shams SF, Jaafari MR (2016) Perspective of Fe3O4 nanoparticles role in biomedical applications. Biochem Res Int. https://doi.org/10.1155/2016/7840161
Sangaiya P, Jayaprakash R (2018) A review on iron oxide nanoparticles and their biomedical applications. J Supercond Novel Magn 31:3397–3413. https://doi.org/10.1007/S10948-018-4841-2
Ganapathe LS, Mohamed MA, Yunus RM, Berhanuddin DD (2020) Magnetite (Fe3O4) nanoparticles in biomedical application: from synthesis to surface functionalisation. Magnetochemistry 6:68. https://doi.org/10.3390/MAGNETOCHEMISTRY6040068
Sharma RK, Dutta S, Sharma S et al (2016) Fe3O4 (iron oxide)-supported nanocatalysts: synthesis, characterization and applications in coupling reactions. Green Chem 18:3184–3209. https://doi.org/10.1039/C6GC00864J
Gawande MB, Branco PS, Varma RS (2013) Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies. Chem Soc Rev 42:3371–3393. https://doi.org/10.1039/C3CS35480F
Shifrina ZB, Bronstein LM (2018) Magnetically recoverable catalysts: beyond magnetic separation. Front Chem 6:298. https://doi.org/10.3389/FCHEM.2018.00298
Ziarani GM, Kheilkordi Z, Mohajer F et al (2021) Magnetically recoverable catalysts for the preparation of pyridine derivatives: an overview. RSC Adv 11:17456–17477. https://doi.org/10.1039/D1RA02418C
Zhang Q, Su H, Luo J, Wei Y (2012) A magnetic nanoparticle supported dual acidic ionic liquid: a “quasi-homogeneous” catalyst for the one-pot synthesis of benzoxanthenes. Green Chem 14:201–208. https://doi.org/10.1039/C1GC16031A
Nguyen HT, Le Thi NP, Nguyen Chau DK, Tran PH (2018) New nano-Fe3O4-supported Lewis acidic ionic liquid as a highly effective and recyclable catalyst for the preparation of benzoxanthenes and pyrroles under solvent-free sonication. RSC Adv 8:35681–35688. https://doi.org/10.1039/C8RA04893B
Nguyen TT, Le Thi NP, Nguyen TT, Tran PH (2019) An efficient multicomponent synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles catalyzed by a magnetic nanoparticle supported Lewis acidic deep eutectic solvent. RSC Adv 9:38148–38153. https://doi.org/10.1039/C9RA08074K
Mohammadi R, Esmati S, Gholamhosseini-Nazari M, Teimuri-Mofrad R (2018) Synthesis and characterization of a novel Fe3O4@SiO2–BenzIm-Fc[Cl]/BiOCl nano-composite and its efficient catalytic activity in the ultrasound-assisted synthesis of diverse chromene analogs. New J Chem 43:135–145. https://doi.org/10.1039/C8NJ04938F
Wang S-L, Ding J, Shi F et al (2012) Green synthesis of 3-hydroxynaphthalene-1,4-dione derivatives via microwave-assisted three-component reactions in neat water. J Heterocycl Chem 49:521–525. https://doi.org/10.1002/JHET.798
López LIL (2014) Naphthoquinones: biological properties and synthesis of lawsone and derivatives—a structured review. Vitae 21:248–258
Jordão AK, Vargas MD, Pinto AC et al (2015) Lawsone in organic synthesis. RSC Adv 5:67909–67943. https://doi.org/10.1039/C5RA12785H
Zhao Z, Dai X, Li C et al (2020) Pyrazolone structural motif in medicinal chemistry: retrospect and prospect. Eur J Med Chem 186:111893. https://doi.org/10.1016/J.EJMECH.2019.111893
Lakshmanan S, Ramalakshmi N (2016) One-pot, four-component synthesis of benzylpyrazolyl naphthoquinone derivatives and molecular docking studies. Synth Commun 46:2045–2052. https://doi.org/10.1080/00397911.2016.1244693
Kumar M, Sribalan R, Padmini V (2017) Er(OTf)3 assisted efficient synthesis of 3-hydroxynaphthalene-1,4-dione derivatives via pseudo four-component reactions and their biological evaluation. Chem Sel 2:489–493. https://doi.org/10.1002/SLCT.201601340
Vairaperumal V, Perumal M, Sengodu P et al (2019) V2O5-catalyzed one-pot multicomponent of pyrazol naphthoquinone as scaffolds for potential bioactive compounds: synthesis, structural study and cytotoxic activity. Chem Sel 4:3006–3010. https://doi.org/10.1002/SLCT.201803942
Patil A, Gajare S, Rashinkar G, Salunkhe R (2019) β-CD-SO3H: synthesis, characterization and its application for the synthesis of benzylpyrazolyl naphthoquinone and pyrazolo pyranopyrimidine derivatives in water. Catal Lett 150:127–137. https://doi.org/10.1007/S10562-019-02928-Y
Fu Z, Qian K, Li S et al (2016) MgCl2 catalyzed one-pot synthesis of 2-hydroxy-3-((5-methyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)(phenyl)methyl)naphthalene-1,4-dione derivatives in EG. Tetrahedron Lett 57:1104–1108. https://doi.org/10.1016/J.TETLET.2016.01.089
Dashteh M, Safaiee M, Baghery S, Zolfigol MA (2019) Application of cobalt phthalocyanine as a nanostructured catalyst in the synthesis of biological henna-based compounds. Appl Organomet Chem 33:e4690. https://doi.org/10.1002/AOC.4690
Yarie M, Zolfigol MA, Babaee S et al (2018) Catalytic application of a nano-molten salt catalyst in the synthesis of biological naphthoquinone-based compounds. Res Chem Intermed 44:2839–2852. https://doi.org/10.1007/S11164-018-3264-9
Kumbhar A, Kamble S, Jadhav S et al (2012) Silica tethered Pd–DABCO complex: an efficient and reusable catalyst for Suzuki-Miyaura reaction. Catal Lett 142(11):1388–1396. https://doi.org/10.1007/S10562-012-0912-3
Kurane R, Bansode P, Khanapure S et al (2016) Intermolecular C-O coupling using hemicucurbituril supported ionic liquid phase catalyst. Catal Lett 146:2485–2494. https://doi.org/10.1007/S10562-016-1871-X/FIGURES/5
Naikwade A, Jagadale M, Kale D et al (2018) Magnetic nanoparticle decorated N-heterocyclic carbene-nickel complex with pendant ferrocenyl group for C-H arylation of benzoxazole. Catal Lett 148(10):3178–3192. https://doi.org/10.1007/S10562-018-2514-1
Kukade MG, Gavade NL, Kadam AN et al (2019) Magnetically retrievable cobalt ferrite nanoparticles as heterogeneous catalyst for synthesis of 1-oxo-hexahydroxanthenes. Asian J Org Med Chem 4:113–120. https://doi.org/10.14233/AJOMC.2019.AJOMC-P207
Gajare S, Patil A, Kale D et al (2019) Graphene oxide-supported ionic liquid phase catalyzed synthesis of 3,4-dihydro-2H-naphtho[2,3-e][1,3]oxazine-5,10-diones. Catal Lett 150(1):243–255. https://doi.org/10.1007/S10562-019-02934-0
Pawar A, Gajare S, Jagdale A et al (2021) Supported NHC-BenzImi@Cu complex as a magnetically separable and reusable catalyst for the multicomponent and click synthesis of 1,4-disubstituted 1,2,3-triazoles via Huisgen 1,3-dipolar cycloaddition. Catal Lett. https://doi.org/10.1007/S10562-021-03772-9
Chandane W, Gajare S, Kagne R et al (2022) Sulfated tin oxide (SO4−2/SnO2): an efficient heterogeneous solid superacid catalyst for the facile synthesis of 2,3-dihydroquinazolin-4(1H)-ones. Res Chem Intermed 2022:1–18. https://doi.org/10.1007/S11164-022-04670-4
Zhao X, Shi Y, Wang T et al (2008) Preparation of silica-magnetite nanoparticle mixed hemimicelle sorbents for extraction of several typical phenolic compounds from environmental water samples. J Chromatogr A 1188:140–147. https://doi.org/10.1016/J.CHROMA.2008.02.069
Liu Q, Xu Z, Finch JA, Egerton R (1998) A novel two-step silica-coating process for engineering magnetic nanocomposites. Chem Mater 10:3936–3940. https://doi.org/10.1021/CM980370A
Naikwade AG, Jagadale MB, Kale DP et al (2020) Photocatalytic degradation of methyl orange by magnetically retrievable supported ionic liquid phase photocatalyst. ACS Omega 5:131–144. https://doi.org/10.1021/ACSOMEGA.9B02040
Dadhania HN, Raval DK, Dadhania AN (2015) Magnetically retrievable magnetite (Fe3O4) immobilized ionic liquid: an efficient catalyst for the preparation of 1-carbamatoalkyl-2-naphthols. Catal Sci Technol 5:4806–4812. https://doi.org/10.1039/C5CY00849B
Togashi T, Naka T, Asahina S et al (2011) Surfactant-assisted one-pot synthesis of superparamagnetic magnetite nanoparticle clusters with tunable cluster size and magnetic field sensitivity. Dalton Trans 40:1073–1078. https://doi.org/10.1039/C0DT01280G
Kooti M, Afshari M (2012) Phosphotungstic acid supported on magnetic nanoparticles as an efficient reusable catalyst for epoxidation of alkenes. Mater Res Bull 47:3473–3478. https://doi.org/10.1016/J.MATERRESBULL.2012.07.001
Zolfigol MA, Ayazi-Nasrabadi R (2016) Synthesis of the first magnetic nanoparticles with a thiourea dioxide-based sulfonic acid tag: application in the one-pot synthesis of 1,1,3-tri(1H-indol-3-yl) alkanes under mild and green conditions. RSC Adv 6:69595–69604. https://doi.org/10.1039/C6RA11620E
Miyatake K, Iyotani H, Yamamoto K, Tsuchida E (1996) Synthesis of poly(phenylene sulfide sulfonic acid) via poly(sulfonium cation) as a thermostable proton-conducting polymer. Macromolecules 29:6969–6971. https://doi.org/10.1021/MA960768X
Langner R, Zundel G (2002) FT-IR investigation of polarizable, strong hydrogen bonds in sulfonic acid sulfoxide, phosphine oxide, and arsine oxide complexes in the middle- and far-infrared region. J Phys Chem 99:12214–12219. https://doi.org/10.1021/J100032A025
Panwar V, Cha K, Park JO, Park S (2012) High actuation response of PVDF/PVP/PSSA based ionic polymer metal composites actuator. Sens Actuators B 161:460–470. https://doi.org/10.1016/J.SNB.2011.10.062
Saikia M, Saikia L (2016) Sulfonic acid-functionalized MIL-101(Cr) as a highly efficient heterogeneous catalyst for one-pot synthesis of 2-amino-4H-chromenes in aqueous medium. RSC Adv 6:15846–15853. https://doi.org/10.1039/C5RA28135K
Ying A, Hou H, Liu S et al (2016) Ionic modified TBD supported on magnetic nanoparticles: a highly efficient and recoverable catalyst for organic transformations. ACS Sustain Chem Eng 4:625–632. https://doi.org/10.1021/ACSSUSCHEMENG.5B01757
Ghosh S, Badruddoza AZ, Uddin MS, Hidajat K (2011) Adsorption of chiral aromatic amino acids onto carboxymethyl-β-cyclodextrin bonded Fe(3)O(4)/SiO(2) core–shell nanoparticles. J Colloid Interface Sci 354:483–492. https://doi.org/10.1016/J.JCIS.2010.11.060
Fakhraian H, Nafari Y (2021) Preparative, mechanistic and tautomeric investigation of 1-phenyl and 1-methyl derivative of 3-methyl-5-pyrazolone. J Chem Sci 133(2):1–11. https://doi.org/10.1007/S12039-021-01902-9
Acknowledgements
We gratefully acknowledge the IIT, Bombay, NEHU, Shillong, IIT, Madras, IISc, Bangalore, Sophisticated Analytical Instrumental Facility and Common Facility Center (CFC) and Physics Instrumentation Facility Centre (PIFC), Shivaji University, Kolhapur for providing spectral facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chandane, W., Gajare, S., Patil, A. et al. Nanoparticle Supported Bronsted Acidic Ionic Liquid Catalyzed Synthesis of Dihydro-1H-pyrazolylnaphthalene-1,4-diones. Catal Lett 153, 3357–3370 (2023). https://doi.org/10.1007/s10562-022-04243-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-022-04243-5