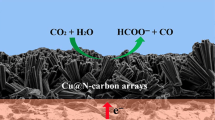
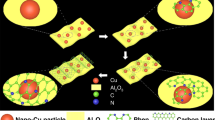
Abstract
Metal-support interaction has been used for selectivity control towards electro-chemical CO2 reduction reaction (CO2RR). However, little progress has been made in adjusting the size of metal nanoparticles in supporting material and its effect on CO2RR. Herein, we prepared Cu metal nanoparticle catalysts ranging from a few nanometers to tens in crystallite size encapsulated in Zn, N-doped carbon supports and systematically study the size effect of the supported Cu metal catalysts on the reactivity of CO2RR. The catalyst containing 15.7-nm Cu crystallites has a higher Faradaic efficiency (nearly 80%) at − 0.7 V vs. RHE for CO, higher than 12.2-nm and 8.9-nm Cu catalysts. The result shows the dependence of CO2RR performance on Cu metal size, originating from the interaction between metal nanoparticles and their supporting matrix. This work may shed light on the promise of in situ construction method for supported metal catalyst and the investigation of metal-support interaction towards stable and selective electrochemical CO2 reduction.
Graphical Abstract
Cu metal nanoparticle catalysts ranging from a few to tens of nanometers in crystallite size were encapsulated in Zn, N-doped carbon supports, of which the size effect and metal-support interaction regulates the reactivity of CO2 electroreduction.






Similar content being viewed by others
References
Zhu Z, Li Z, Wang J et al (2022) Improving NiNX and pyridinic N active sites with space-confined pyrolysis for effective CO2 electroreduction. eScience. https://doi.org/10.1016/j.esci.2022.05.002
Guo Hui, Si D, Zhu H et al (2022) Ni single-atom sites supported on carbon aerogel for highly efficient electroreduction of carbon dioxide with industrial current densities. eScience 2:295–303
Han J, Wang N, Li X et al (2022) Bioinspired iron porphyrins with appended poly-pyridine/amine units for boosted electrocatalytic CO2 reduction reaction. eScience. https://doi.org/10.1016/j.esci.2022.06.003
Huang Y, Mao XN, Yuan GT et al (2021) Size-dependent selectivity of electrochemical CO2 reduction on converted In2O3 nanocrystals. Angew Chem Int Ed 60:15844–15848
Kim J, Choi W, Park JW et al (2019) Branched copper oxide nanoparticles induce highly selective ethylene production by electrochemical carbon dioxide reduction. J Am Chem Soc 141:6986–6994
Chen CB, Li YF, Yu S et al (2020) Cu–Ag tandem catalysts for high-rate CO2 electrolysis toward multicarbons. Joule 4:1688–1699
Sun H, Chen L, Xiong LK et al (2021) Promoting ethylene production over a wide potential window on Cu crystallites induced and stabilized via current shock and charge delocalization. Nat Commun 12:6823
Li RZ, Wang DS (2022) Understanding the structure–performance relationship of active sites at atomic scale. Nano Res. https://doi.org/10.1007/s12274-022-4371-x
Hao Y, Hu F, Chen Y et al (2022) Recent progress of electro-spun nanofibers for zinc-air batteries. Adv Fiber Mater 4:185–202
Hu F, Yu DS, Ye M et al (2022) Lattice-epitaxially formed mesoporous transition metal oxide heterostructures advance water splitting by active Fe-O-Cu bridges. Adv Energy Mater 12:2200067
Van Deelen TW, Hernández Mejía C, de Jong KP (2019) Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat Catal 2:955–970
Wang J, Du CF, Xue Y et al (2021) MXenes as a versatile platform for reactive surface modification and superior sodium-ion storages. Exploration 1:20210024
Chen YJ, Wang PX, Hao HG et al (2021) Thermal atomization of platinum nanoparticles into single atoms: an effective strategy for engineering high-performance nanozymes. J Am Chem Soc 143:18643–18651
Chen SH, Li WH, Jiang WJ et al (2022) MOF encapsulating N-heterocyclic Carbene-ligated copper single-atom site catalyst towards efficient methane electrosynthesis. Angew Chem Int Ed 61:e202114450
Wang G, He CT, Huang R et al (2020) Photoinduction of Cu single atoms decorated on UiO-66-NH2 for enhanced photocatalytic reduction of CO2 to liquid fuels. J Am Chem Soc 142:19339–19345
Choi C, Cheng T, Espinosa MF et al (2019) A highly active star decahedron Cu nanocatalyst for hydrocarbon production at low overpotentials. Adv Mater 31:1805405
Peterson AA, Abild-Pedersen F, Studt F et al (2010) How Cu catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels? Energy Environ Sci 3:41311–41315
Birdja YY, Perez-Gallent E, Figueiredo MC et al (2019) Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat Energy 4:732–745
Reske R, Mistry H, Behafarid F et al (2014) Particle size effects in the catalytic electro-reduction of CO2 on Cu nanoparticles. J Am Chem Soc 136:6978–6986
Zhang ZY, Chi MF, Veith GM et al (2016) Rational design of Bi nanoparticles for efficient electrochemical CO2 reduction: the elucidation of size and surface condition effects. ACS Catal 6:6255–6264
Rahaman M, Dutta A, Broekmann P (2017) Size-dependent activity of palladium nano-particles: efficient conversion of CO2 into formate at low overpotentials. Chemsuschem 10:1733–1741
Jeon HS, Sinev I, Scholten F et al (2018) Operando evolution of the structure and oxidation state of size-controlled Zn nanoparticles during CO2 electro-reduction. J Am Chem Soc 140:9383–9386
Castelo-Quiben J, Abdelwahab A, Perez-Cadenas M et al (2018) Carbon-iron electro-catalysts for CO2 reduction: the role of the iron particle size. J Catal 24:240–249
Li ZD, He D, Yan XX et al (2020) Size-dependent nickel-based electrocatalysts for selective CO2 reduction. Angew Chem Int Ed 59:18572–18577
Gao D, Arán-Ais RM, Jeon HS et al (2019) Rational catalyst and electrolyte design for CO2 electroreduction towards multi-carbon products. Nat Catal 2:198210
Nitopi S, Bertheussen E, Scott SB et al (2019) Progress and per-spectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem Rev 119:7610–7672
Huang HJ, Yu DS, Hu F et al (2022) Clusters induced electron redistribution to tune oxygen reduction activity of transition metal single-atom for metal-air batteries. Angew Chem Int Ed 61:e202116068
Park KS, Ni Z, Côté AP et al (2006) Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. P Natl Acad Sci USA 103:10186–10191
Zhao CM, Dai XY, Yao T et al (2017) Ionic exchange of metal organic frameworks to access single nickel sites for efficient electroreduction of CO2. J Am Chem Soc 139:8078–8081
Guo YC, Feng L, Wu CC et al (2020) Confined pyrolysis transformation of ZIF-8 to hierarchically ordered porous Zn-N-C nanoreactor for efficient CO2 photoconversion under mild conditions. J Catal 390:213–223
Sha YF, Zhang JL, Cheng XY et al (2022) Anchoring ionic liquid in copper electrocatalyst for improving CO2 conversion to ethylene. Angew Chem Int Ed 61:e202200039
Wang G, Huang R, Zhang JW et al (2021) Synergistic modulation of the separation of photo-generated carriers via engineering of dual atomic sites for promoting photocatalytic performance. Adv Mater 33:2105904
Grosse P, Gao DF, Scholten F et al (2018) Dynamic changes in the structure, chemical state and catalytic selectivity of Cu nanocubes during CO2 electroreduction: size and support effects. Angew Chem Int Ed 57:6192–6197
Zhen SY, Zhang G, Cheng DF et al (2022) Nature of the active sites of copper zinc catalysts for carbon dioxide electro-reduction. Angew Chem Int Ed 61:e202201913. https://doi.org/10.1002/anie.202201913
Tang C, Shi JJ, Bai XW et al (2020) CO2 reduction on copper’s twin boundary. ACS Catal 10:2026–2032
Koshy DM, Chen SC, Lee DU et al (2020) Understanding the origin of highly selective CO2 electroreduction to CO on Ni, N-doped carbon catalysts. Angew Chem Int Ed 59:4043–4050
Mao JJ, He CT, Pe JJ et al (2018) Accelerating water dissociation kinetics by isolating cobalt atoms into ruthenium lattice. Nat Commun 9:4958
Ren WH, Tan X, Yang WF et al (2019) Isolated diatomic Ni-Fe metal-nitrogen sites for synergistic electroreduction of CO2. Angew Chem Int Ed 58:6972–6976
Wang CH, Liang JW, Luo J et al (2021) A universal wet chemistry synthesis of solid-state halide electrolytes for all-solid-state lithium-metal batteries. Sci Adv. https://doi.org/10.1126/sciadv.abh1896
Rong WF, Zou HY, Zang WJ et al (2021) Size-dependent activity and selectivity of atomic-level copper nanoclusters during CO/CO2 electroreduction. Angew Chem Int Ed 60:466–472
Handoko AD, Wei F, Yeo BS et al (2018) Understanding heterogeneous electrocatalytic carbon dioxide reduction through operando techniques. Nat Catal 1:922–934
Ju W, Bagger A, Hao GP et al (2017) Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO2. Nat Commun 8:944
Liu X, Schlexer P, Xiao J et al (2019) pH effects on the electrochemical reduction of CO2 towards C2 products on stepped copper. Nat Commun 10:32
Jiang K, Siahrostami S, Zheng T et al (2018) Isolated Ni single atoms in graphene nano-sheets for high-performance CO2 reduction. Energy Environ Sci 11:893–903
Chen Y, Hu F, Hao Y et al (2022) In situ construction of thiol-silver interface for selectively electrocatalytic CO2 reduction. Nano Res 15:3283–3289
Huang JJ, Chen SX, Yang FQ et al (2022) Nickel nanoparticles with narrow size distribution confined in nitrogen-doped carbon for efficient reduction of CO2 to CO. Catal Lett 152:600–609
Zhang XC, Ren GM, Zhang GM et al (2020) Photocatalytic reduction of CO2 to CO over 3D Bi2MoO6 microspheres: simple synthesis, high efficiency and selectivity, reaction mechanism. Catal Lett 150:2510–2516
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 22101132), the Natural Science Foundation of Jiangsu Province (BK20210311), China Postdoctoral Science Foundation (Grant Nos. 2021M691561, 2021T140319) Jiangsu Planned Projects for Postdoctoral Research Fund (2021K547C), CAS Key Laboratory of Nano-Bio Interface (21NBI02), the Fundamental Research Funds for the Central Universities (NS2021037), and the Graduate Innovation Foundation of Nanjing University of Aeronautics and Astronautics (XCXJH20210606). The authors gratefully acknowledge the financial support by the Agency for Science, Technology and Research (A*STAR) under its Career Development Fund (CDF 202D800033).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, H., Hao, Y., Sun, Y. et al. Size Control of Zn, N-doped Carbon Supported Copper Nanoparticles for Effective and Selective CO2 Electroreduction. Catal Lett 153, 2115–2124 (2023). https://doi.org/10.1007/s10562-022-04125-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-022-04125-w