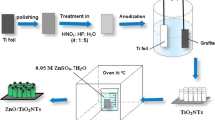
Abstract
Regular TiO2 nanotube arrays are efficient photoanode materials for photocatalytic water splitting for hydrogen evolution. Herein, Cu2O nanoparticles were electrochemically deposited onto TiO2 nanotube surfaces to improve the photocatalytic performance of TiO2 nanotubes (NTbs). The doping amount of Cu2O was regulated by adjusting the pH of the electrolyte during the deposition process. The physicochemical properties of series Cu2O–TiO2 NTbs were characterized. The results show that the deposited Cu2O particles did not destroy the microstructure of the TiO2 NTbs and were uniformly loaded on both the inner and outer walls of the TiO2 NTbs. The as-prepared Cu2O–TiO2 NTbs were used as photoanodes for water-splitting hydrogen evolution. The calculated theoretical hydrogen production of the Cu2O–TiO2 NTbs was 18.66 μmol cm−2 h−1, which was three times higher than that of pure TiO2 NTbs. The photoelectric conversion efficiency was 22.6%. The high efficiency of the catalyst is attributed to the doping of Cu2O nanoparticles, which lowers the recombination rate of photogenerated electron–hole pairs in the Cu2O–TiO2 NTbs, thus improving the photocatalytic performance.
Graphical Abstract







Similar content being viewed by others

References
Bowker M, O’Rourke C, Mills A (2022) The role of metal nanoparticles in promoting photocatalysis by TiO2. Top Curr Chem 17:380
Zhou Y, Sun M (2022) Considering photocatalytic activity of Cu2+/biochar-doped TiO2 using corn straw as sacrificial agent in water decomposition to hydrogen. Environ Sci Pollut Res 29:12261–12281
Jiang YH, Pang HJ, Sun X et al (2021) TiO2 nanobelts with ultra-thin mixed C/SiOx coating as high-performance photo/photoelectrochemical hydrogen evolution materials. Appl Surf Sci 537:147861
Huang Q, Wang C, Hao D et al (2021) Ultralight biodegradable 3D-g-C3N4 aerogel for advanced oxidation water treatment driven by oxygen delivery channels and triphase interfaces. J Clean Prod 288:125091
Du Y, Hao Q, Chen DM et al (2017) Facile fabrication of heterostructured bismuth titanate nanocomposites: the effects of composition and band gap structure on the photocatalytic activity performance. Catal Today 297:255–263
Hao D, Liu Y, Gao SY et al (2021) Emerging artificial nitrogen cycle processes through novel electrochemical and photochemical synthesis. Mater Today 46:212–233
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nat 238:37–38
Zhao C, Zhu DC, Cao SX (2016) Amorphous TiO2 nanotube-derived synthesis of highly ordered anatase TiO2 nanorod arrays. Superlattice Microst 90:257–264
Momeni MM, Ghayeb Y, Ghonchegi Z (2015) Fabrication and characterization of copper doped TiO2 nanotube arrays by in situ electrochemical method as efficient visible-light photocatalyst. Ceram Int 41:8735–8741
Momeni MM (2015) Fabrication of copper decorated tungsten oxide–titanium oxide nanotubes by photochemical deposition technique and their photocatalytic application under visible light. Appl Surf Sci 357:160–166
Momeni MM, Ghayeb Y, Ezati F (2018) Fabrication, characterization and photoelectrochemical activity of tungsten-copper co-sensitized TiO2 nanotube composite photoanodes. J Colloid Interf Sci 514:70–82
Han J, Hou XJ, Liu HL et al (2019) Photocurrent enhancement on TiO2 nanotubes co-modified by N+ implantation and combustion of grapheme. Mater Lett 238:77–80
Masegi H, Doi N, Yoneyama S et al (2021) Visible light responsive photocatalytic hydrogen production over composites of anodized TiO2 nanotube array and graphitic carbon nitride measured with a gas circulating reactor. Jpn J Appl Phys 60:105504
Javed F, Javed S, Mujahid M et al (2019) Modified optical characteristics of TiO2/Au/TiO2 thin composite films. Ceram Int 45:22336–22343
She HD, Ma X, Chen KY et al (2020) Photocatalytic H2 production activity of TiO2 modified by inexpensive Cu(OH)2 cocatalyst. J Alloy Compd 821:153239
Zhen WL, Jiao WJ, Wu YQ et al (2017) The role of a metallic copper interlayer during visible photocatalytic hydrogen generation over a Cu/Cu2O/Cu/TiO2 catalyst. Catal Sci Technol 7:5028–5037
Wang XQ, Dai M, Chen QH et al (2021) Enhanced photoelectrochemical performance of NiO-doped TiO2 nanotubes prepared by an impregnation–calcination method. J Chem Res 45:1076–1082
Moon HS, Yong KJ (2020) Noble-metal free photocatalytic hydrogen generation of CuPc/TiO2 nanoparticles under visible-light irradiation. Appl Surf Sci 530:147215
Wang JH, Zhu HF, Tang SH et al (2020) Sandwich structure MoO2/MoS2/TiO2 photocatalyst for superb hydrogen evolution. J Alloy Compd 842:155869
Li GJ, Huang JQ, Chen J et al (2019) Highly Active Photocatalyst of Cu2O/TiO2 Octahedron for Hydrogen Generation. ACS Omega 4:3392–3397
Wang JY, Ji GB, Liu YS et al (2014) Cu2O/TiO2 heterostructure nanotube arrays prepared by an electrodeposition method exhibiting enhanced photocatalytic activity for CO2 reduction to methanol. Catal Commun 46:17–21
Benz D, Nguyen YN, Le TL et al (2021) Controlled growth of ultrasmall Cu2O clusters on TiO2 nanoparticles by atmospheric-pressure atomic layer deposition for enhanced photocatalytic activity. Nanotechnology 32:425601
Deng C, Hong RJ, Jing M et al (2019) Photocatalytic performance of TiO2 thin film decorated with Cu2O nanoparticles by laser ablation. Opt Mater 94:130–137
Acknowledgements
This work was supported by the National Natural Science Foundation of China [Grant Number 21905042]. We are also grateful for the measurement assistants from the Analysis & Testing Center of Northeast Petroleum University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Qiao, P., Chen, Q. et al. Electrochemically Deposited Cu2O-Doped TiO2 Nanotube Photoanodes for Hydrogen Evolution. Catal Lett 153, 1689–1695 (2023). https://doi.org/10.1007/s10562-022-04121-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-022-04121-0