Abstract
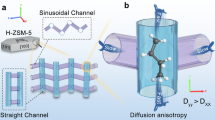
Diffusion process plays a decisive role in MTO reactions over zeolite catalysts. In this work, a theoretical approach was developed for modelling olefins diffusion in two typical zeolites, HZSM-5 and HSAPO-34. Activation barrier between large cavities and channels was determined using Lennard–Jones (LJ) potentials, where electrostatic potential was inserted to account for the induced dipole force that had been ignored in previous studies. Six typical products of MTO were selected as probe molecules. Detailed insights into the variation of activation barrier and diffusivity were obtained via comparative analysis between the two zeolites. Transition from Knudsen diffusion to configurational diffusion was also discriminated, and probe molecules were found to fall basically either in configurational regime or near transition regime. This work provides a submodel for further modeling of the complete reaction system, and ultimately contributes to a rational design of zeolite catalysts.
Graphic Abstract











Similar content being viewed by others
Abbreviations
- d c :
-
Channel and window diameter inside HZSM-5 and HSAPO-34, Å
- d i :
-
Cage and intersection diameter of HZSM-5 and HSAPO-34, Å
- d m :
-
Molecular kinetic diameter, Å
- d o :
-
Oxygen diameter, Å
- d w :
-
Distance from window/channel wall, Å
- D :
-
Intracrystalline diffusivity, M2/s
- D k :
-
Knudsen diffusion coefficient, m2/s
- E a :
-
Activation Energy, kJ/mole
- f :
-
Orientation probability coefficient
- k :
-
Boltzmann Constant, J/K
- L :
-
Length of channel and window, m
- M :
-
Molecular mass, kg
- q :
-
Charge on hydrogen of bronsted site, eV
- r :
-
Distance between guest molecule and native site, Å
- R:
-
Ideal gas constant, J/mol. K
- R c :
-
Distance between centres of channel/window and nuclei of surface oxygen, Å
- R i :
-
Distance between centres of intersection/cage and nuclei of surface oxygen, Å
- T :
-
Temperature, K
- V c :
-
Potential in window/channel of HSAPO-34 and HZSM-5, kJ/mole
- V e :
-
Electrostatic potential, kJ/mole
- V i :
-
Potential inside cage/intersection of HSAPO-34 and HZSM-5, kJ/mole
- Z :
-
Coordination number of zeolites
- α:
-
Mean molecular polarizability, Å3
- ε:
-
Permittivity of medium, for zeolite it is almost equal to 1
- εo :
-
Vacuum permittivity
- μ:
-
Dipole moment, C. m
- \(\overline{V}\) :
-
Molecular diffusional mean velocity, m/s
- εm :
-
Lennard Jones potential constant for individual molecule, KJ/mole
- εmo :
-
Lennard Jones potential constant for molecule-oxygen pair, KJ/mole
- σc :
-
σI, Pairwise Lennard Jones potential length constant between molecule-oxygen pair inside channel/window, Å
- σm :
-
Lennard Jones length constant, Å
References
Primo A, Garcia H (2014) Chem Soc Rev 43:7548
Chen JQ, Bozzano A, Glover B, Fuglerud T, Kvisle S (2005) Catal Today 106:103
Arora SS, Shi Z, Bhan A (2019) ACS Catal 6407.
Liu Y, Kirchberger FM, Müller S, Eder M, Tonigold M, Sanchez-Sanchez M, Lercher JA (2019) Nat Commun 10.
Nasser GA, Muraza O, Nishitoba T, Malaibari Z, Yamani ZH, Al-Shammari TK, Yokoi T (2019) Ind Eng Chem Res 58:60
Huang X, Li H, Li H, Xiao WD (2017) AlChE J 63:306
Hwang A, Le TT, Shi Z, Dai H, Rimer JD, Bhan A (2019) J Catal 122.
Shang Y, Wang W, Zhai Y, Song Y, Zhao X, Ma T, Wei J, Gong Y (2019) Microporous Mesoporous Mater 276:173
Ali MA, Al-Baghli NA, Nisar M, Malaibari ZO, Abutaleb A, Ahmed S (2019) Energy Fuels 33:1458
Olsbye U, Svelle S, Bjrgen M, Beato P, Janssens TVW, Joensen F, Bordiga S, Lillerud KP (2012) Angew Chem Int Ed 51:5810
Zhai Y, Zhang S, Shang Y, Song Y, Wang W, Ma T, Zhang L, Gong Y, Xu J, Deng F (2019) Catal Sci Technol 9:659
Masuda T (2003) Catal Surv Asia 7:133
Lobo RF, in: Ordered Porous Solids, eds. V. Valtchev, S. Mintova, M. Tsapatsis (Elsevier, Amsterdam, 2009)
Xiao J, Wei J (1992) Chem Eng Sci 47:1143
Xiao J, Wei J (1992) Chem Eng Sci 47:1123
Wang C, Li B, Wang Y, Xie Z (2013) J Energy Chem 22:914
Galliéro G, Boned C, Baylaucq A, Montel F (2006) Phys Rev E 73:061201
Bird RB, Stewart WE, Lightfoot EN (2001) Transport Phenomena. Wiley, New York
Keil FJ, Hinderer J, Garayhi AR (1999) Catal Today 50:637
Kulprathipanja S (2010) Zeolites in Industrial Separation and Catalysis. WILEY-VCH, Weinheim
Reid RC, Prausnitz JM, Poling BE (1987) The Properties of Gases and Liquids. McGraw-Hill, New York
Rappe AK, Goddard WA (1991) J Phys Chem 95:3358
Zecchina A, Lamberti C, Bordiga S (1998) Catal Today 41:169
Zalden P, Song L, Wu X, Huang H, Ahr F, Mücke OD, Reichert J, Thorwart M, Mishra PK, Welsch R, Santra R, Kärtner FX, Bressler C (2018) Nat Commun 9:1–7
Miller KJ (1990) J Am Chem Soc 112:8533
Poier PP, Jensen F (2019) J Chem Theory Comput 15:3093
Mayer A, Åstrand PO (2008) J Phys Chem A 112:1277
Wu W, Guo W, Xiao W, Luo M (2013) Fuel Process Technol 108:19
Huang X, Aihemaitijiang D, Xiao WD (2015) Chem Eng J 280:222
Huang X, Aihemaitijiang D, Xiao WD (2016) Chem Eng J 286:150
Huang X, Li H, Xiao WD, Chen D (2016) Chem Eng J 299:263
Acknowledgements
This work is financially supported by the 2019 Key Technology Project of Inner Mongolia, China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hayat, K., Li, XG. & Xiao, WD. Theoretical Insights into Intracrystalline Diffusion of Olefins in MTO Catalysts. Catal Lett 150, 2056–2067 (2020). https://doi.org/10.1007/s10562-020-03136-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03136-9