Abstract
Kinetics and thermodynamics of \(\alpha\)-pinene oxide isomerization was investigated both theoretically and experimentally in different solvents in the temperature range of 50–140 °C using different zeolites, iron modified zeolites, Fe–H–MCM-41 and micro-mesoporous ZSM-5 derived catalysts. The aim was to elucidate the effect of solvent basicity and polarity on the product distribution in this reaction giving as the main value-added products campholenic aldehyde and trans-carveol, which are used as fragrances and perfumes. A generic kinetic first order model was developed composed of both parallel and consecutive routes. A thermodynamic analysis showed that product selectivity is determined by kinetic control and not by thermodynamics. Formation of campholenic aldehyde was favored in non-polar solvents, whereas basic solvents promoted formation of trans-carveol independent on temperature.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
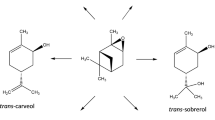
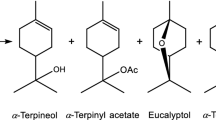
Isomerization of \(\alpha\)-pinene oxide has been intensively studied in the recent years over several homogeneous and heterogeneous catalysts [1,2,3,4,5,6,7,8,9]. The two main valuable products are campholenic aldehyde and trans-carveol finding applications in perfumes and flavors [10]. Isomerization of \(\alpha\)-pinene oxide is a complex reaction including both parallel and consecutive routes (Scheme 1). For production of different compounds it is important to select both optimum reaction conditions, such as solvent and temperature as well as to use a catalyst with the desired properties. Solvent selection can be crucial, since it can be polar or apolar, protic or aprotic [11]. In addition, solvents can be basic or neutral [7]. Typically synthesis of campholenic aldehyde has been performed in neutral solvents at relatively low temperatures, i.e. 70 °C [5, 6]. Furthermore, weakly acidic illite clays promoted formation of fencholenic aldehyde at 30 °C in cyclohexane [12]. On the other hand, trans-carveol was produced using a basic solvent, N,N-dimethylacetamide (DMA) at a higher temperature 140 °C [1, 4]. A rather selective catalyst for trans-carveol (S = 43%) has been, for example Fe–H-beta exhibiting both Brønsted and Lewis acid sites [4], when the reaction was performed in DMA at 140 °C. On the other hand campholenic aldehyde has been formed on Fe–SiO2 in toluene as a solvent [6] and on Fe-Y-12 in toluene [7]. It was, however, observed very recently, that very high selectivity to campholenic aldehyde can be achieved over MCM-22 in \(\alpha\)-pinene oxide isomerization in a basic solvent, N,N-dimethylacetamide at 140 °C [8]. Recently ZSM-5 based micro-mesoporous materials were demonstrated to have moderate selectivity to both campholenic aldehyde and trans-carveol, around 30–50% [3].
Reaction scheme for \(\alpha\)-pinene oxide isomerization adapted from [3]
Effect of solvents has been scarcely investigated in \(\alpha\)-pinene oxide isomerization apart from few studies [2, 7]. Moreover, the specific effects of temperature and solvents are not fully understood, although there is an indication that temperature does not substantially influence \(\alpha\)-pinene oxide isomerization in DMA as a solvent [2] over H3PW12O40 as a catalyst. High selectivity to trans-carveol was reported in [2] where several solvents with varying dielectric coefficients, such as cyclohexane, 1,4-dioxine, N,N-dimethylacetamide and acetone were used. The highest yields of campholenic aldehyde were obtained at 70 °C with cyclohexane as a solvent, whereas both dimethylformamide and N,N-dimethylacetamide afforded 90% and 86% yield respectively trans-carveol at 140 °C [2]. Moreover, in our previous work [7] it was shown that selectivity to trans-carveol increased and selectivity to campholenic aldehyde was decreased systematically with increasing solvent polarity when toluene, acetonitrile, tetrahydrofuran, n-pentanol, N,N-dimethylacetamide and N-methylpyrrolidene were used as solvents in \(\alpha\)-pinene oxide isomerization over Ce–Si–MCM-41 catalysts [7]. In that study, however, only a relatively high reaction temperature, 140 °C was used with DMA and dimethylpyrrolidone (DMP). As a conclusion it can be stated that the product distribution in \(\alpha\)-pinene oxide isomerization is very sensitive to several parameters, such as temperature, catalyst and solvent.
According to our knowledge there is only one study on kinetic analysis and thermodynamic evaluation of \(\alpha\)-pinene oxide isomerization [9]. In that work [9] kinetic modelling was done for experimental data in the temperature range of 298–358 K over Zn-triflate supported on three different supports K60, K100 and hexagonal mesoporous silica HMS24. Thermodynamic analysis was limited to calculations of Gibbs free energy for formation of campholenic aldehyde at 25 °C.
The aim in this work was to compare kinetic results with thermodynamic evaluation, which done by the Joback’s method [13, 14] in order to elucidate the effect of different parameters, such as temperature, solvent polarity and basicity in \(\alpha\)-pinene oxide isomerization. A generic kinetic model was developed for \(~\alpha\)-pinene oxide isomerization and applied for modelling of experimental data made in this work and taken from [5, 6] for a range of iron and iron-free catalysts with different acidity, including Fe–H–MCM-41 [6], mildly acidic H-Beta-300 (the number denotes SiO2/Al2O3 ratio) [6], iron modified H-Beta-300 [5] and micro-mesoporous ZSM-5 [3] based catalysts. The rate constants obtained from kinetic modelling were compared with the initial rates calculated from the kinetic results in order to assess the ability of the generic kinetic model to quantitatively describe the kinetic data.
2 Experimental Section
Isomerization of \(\alpha\)-pinene oxide (97%, Aldrich) was performed in this work with two catalysts, H-Beta-300 and Fe–H-Beta-300. The preparation and characterization of catalysts, H-Beta-300 and Fe–H-Beta-300, used in this work, are reported in [4, 5].
The kinetic experiments were performed in a glass reactor using N,N-dimethylacetamide and toluene as solvents at 323 K and 343 K. In a typical experiment isomerization was performed in a glass reactor equipped with efficient motor stirring and a reflux cooler. The catalyst was heated to 523 K for 30 min in argon atmosphere prior to the reaction. The initial concentration of \(\alpha\)-pinene oxide was 0.013 mol/l and the catalyst amount was 75 mg. The experiments were performed in the kinetic regime with small catalyst particles (below 63 μm) with the stirring speed of 400 rpm. The samples were taken after a certain time intervals (1, 5, 10, 20, 40, 60, 120 and 180 min) and analyzed with a gas chromatograph (HP 6890 series) equipped with a HP-5 column (30 m, 320 μm, 0.50 μm) using the following temperature programme: 333 K (2 min) − 5 K/min − 301 K (15 min) using the split ratio of 20:1. The products were quantified calculating the response factors for the following compounds: campholenic aldehyde (86%, Hangzhou Dayangchem Co Ltd), trans-carveol (95%, SAFC), p-cymene (90%, Fluka) and trans pinocarveol (Aldrich, > 96%). The products were confirmed by GC-MS (Agilent technologies GC-MS 6890N) equipped with DP-5 column (30 m, 320 μm, 0.50 μm).
The initial formation rates of campholenic aldehyde and trans-carveol were calculated during the first 10 min reaction time as follows:
in which r0, i is the initial formation rate of i, t time and mcat catalyst mass.
3 Results and Discussion
3.1 Enthalpy and Gibbs Free Energy Changes for \(\alpha\)-Pinene Oxide Isomerization
Enthalpy (ΔH0r) and Gibbs free energy (ΔG0r) at standard conditions were calculated following a thermodynamic approach [15, 16], starting from the standard enthalpy (ΔH0f) and Gibbs free energy (ΔG0f) of formation from the elements estimated with the Joback approach [13, 14]:
The equilibrium constant of each reaction was calculated from its definition:
Dependence of the reaction free Gibbs energy on temperature was calculated by implementing the Gibbs–Helmholtz equation valid at P = 1 bar (ΔGΦr,j):
The calculated enthalpy and Gibbs free energy formation for each component (i) are reported in Table 1. The stoichiometric matrix was built based on the reaction scheme reported in Scheme 1.
Starting from these values, the enthalpy and Gibbs free energy for each reaction (j) at standard conditions, equilibrium constants at standard conditions (K0j), enthalpy and Gibbs free energy at different temperatures and pressure were calculated. A temperature range of Tmin = 353.15 K and Tmax = 423.15 K was investigated. The results of the calculations are reported in Table 2 and Fig. 1.
Formation of p-cymene from trans-carveol (reaction 7) does not occur spontaneously in the investigated temperature range. The Gibbs energy values for reactions 1 and 2, formation of campholenic and fencholenic aldehyde, are overlapped, as the Joback’s approach does not distinguish among these two products showing the minima Gibbs free energy values, thus the related products are the most favorable to be obtained. At 370 K there is a shift between the product distribution: at T < 370 K the reaction 3, formation of isopinocamphone shows the lower free energy, while reaction 2, formation of fencholenic aldehyde at T > 370 K. The Gibbs free energy for formation of campholenic aldehyde was − 138 kJ/mol being comparative to that given in [9] where Gibbs energy change for formation of campholenic aldehyde calculated using the group contribution method was reported being − 109.35 kJ/mol at 298 K. When comparing the Gibbs free energy for formation of campholenic aldehyde with trans-carveol, it can clearly be seen that even if both are thermodynamically feasible, formation of campholenic aldehyde is even more favorable than trans carveol.
3.2 Qualitative Kinetics
Qualitative kinetics of \(\alpha\)-pinene oxide isomerization was investigated previously by our group over different catalysts including ZSM-5 based micro-mesoporous catalysts (Fig. 2) [3], mesoporous Fe–MCM-41 (Fig. 3), zeolites H-Beta-300 and Fe-Beta-300 (Fig. 4) [4, 5]. These catalysts exhibited different properties (Table 3). Several mesoporous catalysts, such as Al–SBA-15, Fe–H–MCM-41, 7MMAS and 4MMAS were also used to compare different mesopore sizes varying from 2.7 to 25 nm (Table 3, entries 1–4). 7MMAS exhibited also large amounts of strong acid sites. In addition to mesoporous materials also H-Beta-300 and Fe-Beta-300 were applied in this reaction. Among these catalysts Fe–MCM-41 exhibited the lowest acidity. Iron modification typically decreased the Brønsted acidity and increased the Lewis acidity, especially for Fe–MCM-41 (Table 3, entry 4).
Kinetic modelling of \(\alpha\)-pinene oxide isomerization over Al–SBA-15, 7MMAS and 4MMAS catalysts at 413 K in N,N-dimethylacetamide as a solvent. Notation: (o, dark blue) \(\alpha\)-pinene oxide (APO), (diamond, pink) trans-carveol (TCV), (+, red) campholenic aldehyde (CA), (*) fencholenic aldehyde (FA), (pentagon, green) pinocarveol, (inverted triangle, dashed red) 2,8-menthadien-1-ol, (star, dark blue) p-cymene and (hexagon, light blue) other
Kinetic modelling of \(\alpha\)-pinene oxide isomerization over Fe–H–MCM-41 at 343 K in toluene as a solvent. Notation: (o, dark blue) \(\alpha\)-pinene oxide (APO), (diamond, pink) trans-carveol (TCV), (+, red) campholenic aldehyde (CA), (*) fencholenic aldehyde (FA), (pentagon, green) pinocarveol, (inverted triangle, dashed red) pinocamphone, (diamond, dotted, pink) cis carveol, (star, dark blue) p-cymene and (hexagon, light blue) other
Kinetic modelling of \(\alpha\)-pinene oxide isomerization over a H-Beta-300 at 343 K and b at 413 K in DMA, c over Fe–H-Beta-300 at 323 K and d at 343 K in toluene. Notation: (o, dark blue) \(\alpha\)-pinene oxide (APO), (diamond, pink) trans-carveol (TCV), (+, red) campholenic aldehyde (CA), (*) fencholenic aldehyde (FA), (pentagon, green) pinocarveol, (inverted triangle, dashed red) pinocamphone, (diamond, dotted, pink) cis carveol, (star, dark blue) p-cymene and (hexagon, light blue) other
Over these micro-mesoporous ZSM-5 derived catalysts the initial formation rates of campholenic aldehyde and trans-carveol in \(\alpha\)-pinene oxide isomerization in DMA at 140 °C were rather small and there were no large differences in the initial rates for formation of campholenic aldehyde and trans-carveol (Table 4, entries 1–3). The highest initial rate for campholenic aldehyde and trans-carveol among these three catalysts was obtained with a mildly acidic Al–SBA-15. Conversion of \(\alpha\)-pinene oxide was the lowest with 4MMAS exhibiting the smallest mesopores (Table 4, entry 3). These results show that it was possible to regulate the catalytic activity by changing pore structure [3].
Over these micro-mesoporous ZSM-5 derived catalysts only a slightly higher selectivity to trans-carveol were obtained in comparison with campholenic aldehyde at 140 °C in DMA as a solvent (Table 4, entries 1–3, Fig. 2). The third major product over ZSM-5 derived catalysts was 2,8-menthadien-1-ol confirmed by GC–MS [3]. As a comparison, microporous H-Beta-300 gave a quite similar ratio between the selectivities of trans-carveol to campholenic aldehyde as 7MMAS (Table 3, entry 2) despite their different structures. Formation of p-cymene was very minor over these catalysts in DMA as a solvent despite a high reaction temperature, 140 °C and utilization of micro-mesoporous catalysts (Fig. 2).
Mesoporous mildly acidic Fe–MCM-41 was very active transforming \(\alpha\)-pinene oxide to mainly campholenic aldehyde (Table 4, entry 4, Fig. 3). The initial formation rate of campholenic aldehyde was 2.4 fold higher than the initial formation rate of trans-carveol, confirming that formation of campholenic aldehyde was favored in a neutral solvent, toluene at 70 °C. Noteworthy is that with this catalyst also trans-carveol was reacting further to p-cymene (Fig. 3) showing clearly that this type of mesoporous catalyst containing a low amount of Brønsted acid sites is able to catalyze dehydration of trans-carveol. Moreover, the amounts of the formed p-cymene and fencholenic aldehydes are about the same after 180 min over mildly acidic Fe–H–MCM-41.
Fe–H-Beta-300 was also active in \(\alpha\)-pinene oxide isomerization giving the same initial formation rate of campholenic aldehyde as Fe–H–MCM-41 under the same conditions in toluene and high conversion (Fig. 4; Table 4, entry 5). Most probably, the amount of acid sites located outside Beta framework is adequate to catalyze this reaction, since the maximum sphere which can diffuse into Beta structure is 0.595 nm [17], whereas the diameters including van der Waals diameters of \(\alpha\)-pinene oxide, campholenic aldehyde and trans-carveol are 0.71 × 0.83 nm, 0.79 × 1.01 nm and 0.77 × 1.09 nm, respectively [8]. It was also interesting to observe that p-cymene formation was lower in microporous Fe–H-Beta-300 (Fig. 4c, d) than over mesoporous Fe–H–MCM-41 (Fig. 3) most probably due to the pore size, i.e. in the microporous catalyst the consecutive reaction of trans-carveol is less prominent due to steric hindrance.
The effect of temperature was quite prominent between 50 °C and 70 °C over a mildly acidic Fe–H-Beta-300 in \(\alpha\)-pinene oxide isomerization in toluene, since the initial formation rate of campholenic aldehyde and conversion increased from 2.0 mmol/mingcat to 3.6 mmol/mingcat and from 60 to 92%, respectively (Table 4, entries 5, 6).
The effect of temperature was also investigated over H-Beta-300 using a basic DMA as a solvent in \(\alpha\)-pinene oxide isomerization at 70 °C and 140 °C (Table 4, entries 7 and 8). In this case both the initial rate and conversion were increased substantially. On the other hand, it was confirmed that temperature did not have any major effect on product distribution analogously to [2]. The ratio between the initial formation rates for formation of trans-carveol and campholenic aldehyde was 2.25 at 70 °C, whereas it was 1.9 at 140 °C in DMA as a solvent. Noteworthy is also that over H-Beta-300 only traces of p-cymene were formed in DMA as a solvent at 140 °C (Fig. 4a, b).
The use of N,N-dimethylacetamide resulted in a rather high selectivity to trans-carveol even at 70 °C (Table 4, entry 7). The highest selectivity to trans-carveol over H-Beta-300 obtained at 140 °C in DMA was 43% being higher than that for campholenic aldehyde [4]. These results are in line with the work of [2], who obtained high selectivity to trans-carveol in DMA over H3PW12O40 in the temperature range of 25–140 °C. These results confirm that the basic nature of the solvent is decisive for formation of trans-carveol with its formation being independent on temperature. When comparing these results with thermodynamics presented above it can also be stated that high selectivity to trans-carveol can be obtained in \(\alpha\)-pinene oxide isomerization even at a low reaction temperature in a basic solvent even if, from the viewpoint of thermodynamics, formation of campholenic aldehyde is more favourable (Fig. 5).
In order to better understand the role of solvent, temperature and catalyst selection in \(\alpha\)-pinene oxide isomerization, both the initial rate for \(\alpha\)-pinene oxide transformation and the selectivity to trans-carveol were plotted as a function of solvent polarity (Fig. 6) [9]. The initial \(\alpha\)-pinene oxide transformation rate was calculated from the kinetic data presented in this work and in [3,4,5,6,7] by dividing the moles of converted \(\alpha\)-pinene oxide during the first 10 min by consumed time and catalyst mass. The results revealed that the initial transformation rate of \(\alpha\)-pinene oxide decreased with increasing solvent polarity (Fig. 6a). The highest initial rates were obtained at 70 °C with the mildly acidic Fe–H-Beta-300 in toluene followed by Fe–H–MCM-41 at 70 °C in toluene. In DMA much lower initial transformation rates were obtained, although the reaction temperature was usually very high, 140 °C. Noteworthy is also that the initial transformation rate of \(\alpha\)-pinene oxide was very low in DMA at 70 °C over mildly acidic H-Beta-300, although this catalyst exhibited rather high initial rate for \(\alpha\)-pinene oxide transformation at 140 °C. Several solvents were used in \(\alpha\)-pinene oxide isomerization at their boiling points using a mildly acidic composite material, 32 wt% Ce–Si–MCM-41 as a catalyst. The initial rates decreased in this series also with increasing solvent polarity, except for DMA, which gave a very low initial rate despite its polarity.
a Initial rate for transformation of α-pinene oxide over different catalysts, solvents and temperatures as a function of solvent polarity ENT [18], data is from this work and from [3,4,5,6,7], b Selectivity to trans-carveol in α-pinene oxide isomerization as a function of solvent polarity ENT [18]. The data generated in this work are denoted as (circle). In addition to the data from this work (Table 3) and from [3,4,5,6,7]. Notation: DMA N,N-dimethylacetamide, NMP N-methylpyrrolidone, THF tetrahydrofuran
Only time for 100% conversion of \(\alpha\)-pinene oxide was reported for its isomerization over H3PW12O40 at different temperatures [2]. In that work the shortest time for complete conversion of \(\alpha\)-pinene oxide was obtained at 25 °C in cyclohexane and in acetone at 5 min. These solvents exhibit the ENT values of 0.006 and 0.355, respectively, showing that even acetone as a solvent with a relatively high polarity value gave high isomerization rates of \(\alpha\)-pinene oxide [2]. Thus, it can be concluded that the initial transformation rate decreased with increasing solvent polarity with some exceptions, such as DMA with Ce–Si–MCM-41 as a catalyst, which can be related to catalytic properties per se.
In addition to temperature and basicity, selectivity to trans-carveol was also plotted as a function of solvent polarity taken from [9] (Fig. 6b) showing that there was no direct correlation between trans-carveol selectivity and solvent polarity. When comparing the initial transformation rates of \(\alpha\)-pinene oxide (Fig. 6a) and selectivity to trans-carveol (Fig. 6b) it can be stated that, for example, the initial rate was low in THF giving also low selectivity to trans-carveol indicating that there is no direct correlation between a low initial rate and selectivity to trans-carveol.
3.3 Kinetic Modelling
A kinetic model was developed for \(\alpha\)-pinene oxide isomerization in [9] using an integral method. In that work both consecutive and parallel routes were proposed, even if the data fitting was only based on \(\alpha\)-pinene oxide conversion resulting in a zero order model with respect to the reactant concentration.
Kinetic modelling of \(\alpha\)-pinene oxide isomerization in the current study is based on the rate equations, which were derived for the reaction scheme (Scheme 1) assuming a low coverage of reactant on the surface of catalysts:
The corresponding mass balances for the batch reactor for different compounds (with the notation: APO \(\alpha\)-pinene oxide, CA campholenic aldehyde, FA fencholenic aldehyde, TCV trans-carveol, PCP isopinocamphone, PCV pinocarveol, MDI 2,8-menthadien-1-ol, CCV cis-carveol, PCY p-cymene) are:
Over other catalysts selected for kinetic modelling, i.e. H-Beta-300, Fe–H-Beta-300, Fe–H–MCM-41, no 2,8-menthadion-1-ol was found, while cis-carveol was formed [4,5,6,7], which can further react to p-cymene. In this case, the model was modified by taking into account formation of cis-carveol:
and p-cymene
The differential equations were solved using the backward difference method and the parameter estimation was performed with the simplex and Levenberg–Marquardt methods. The numerical tools are built into the used optimization software Modest [19] in which the objective function was defined as
and the degree of explanation R2 is defined as
where \({y_i}\) and \({\widehat {y}_i}\) are experimental and modelled concentrations. The parameter estimation results and modelling are shown in Tables 5, 6, 7, 8, 9 and 10 and Figs. 2, 3 and 4. The model fitting is adequate for all cases (Fig. 2, 3 and 4b, c and d) except for Fig. 4a. In \(\alpha\)-pinene oxide isomerization over Fe–H–MCM-41 the largest error was for k5 for which the original data for trans-carveol are slightly scattered. On the other hand, the highest values for k7 and k9 were correctly estimated for Fe–H–MCM-41 giving the highest amounts of p-cymene (Tables 5, 6, 7, 8) for Fe–H-Beta-300 and H-Beta-300 (Tables 9, 10) low amounts of p-cymene were formed resulting in poor identifiability of the corresponding constants and the error became large. Because of a limited data set for H-Beta-300 varying temperature the errors in k5 and EA5 for formation of trans-carveol were rather high (Table 10).
In order to qualitative compare the values of the initial formation rates of campholenic aldehyde and trans-carveol over different catalysts (Table 4) and the values of k1 and k6 obtained kinetic modelling (Tables 5, 6, 7, 8, 9, 10), they were plotted in a bar diagram (Fig. 7). It can be seen from Fig. 7 that for example in the case of Al–SBA-15 when the reaction was carried out in DMA at 140 °C, r0, CA, calc was smaller than r0, TCV, calc and the same trend was valid for k1,mod. and k6, mod. On the other hand, in a neutral solvent toluene Fe–H–MCM-41 gave higher r0, CA, calc. than k1, mod. analogously to r0, TCV,calc and k6,mod, indicating that qualitative trends with the generic model can be well described. Furthermore, the rates were higher in neutral solvent than in the basic one. At the same time, when the reaction proceeds rapidly, the main product is campholenic aldehyde, whereas in a basic solvent, when the rate is low, the main product was trans-carveol.
Comparison of calculated initial rates for CA and TCV (Table 4) and the modelled k1 and k6 for different catalysts (Tables 4, 5, 6). Notation: Al–SBA-15, 7MMAS and 4MMAS at 140 °C, Fe–H–MCM-41 and Fe–H-Beta-300, and H-Beta-300. Initial rates for Fe–H–MCM-41 and Fe-Beta-300 and H-Beta-300 were calculated at 70 °C. Notation; (1) DMA, (2) toluene as a solvent
4 Conclusions
The effect of the solvent properties, such as basicity and polarity as well as the reaction temperature was investigated in \(\alpha\)-pinene oxide isomerization using Fe–H-Beta-300 and H-Beta-300 catalysts both exhibiting mild acidity. The results showed that main product typically in non-polar solvents, such as toluene, is campholenic aldehyde, whereas trans-carveol formation is favored in basic solvents independent on temperature. The selectivity to trans-carveol was independent on the solvent polarity. The kinetic results were compared with the thermodynamic calculations showing that campholenic aldehyde formation is more feasible than formation of trans-carveol. Dehydration of trans-carveol to p-cymene is thermodynamically not feasible. The kinetic results showed also that product selectivity in \(\alpha\)-pinene oxide isomerization is governed by the kinetic control.
A generic kinetic model for isomerization of \(\alpha\)-pinene oxide containing parallel first order kinetic equations for formation of different primary products from the reactants and a consecutive route for p-cymene formation from trans- and cis-carveol was developed. The kinetic model was describing well the kinetic data for both micro- and mesoporous catalysts. The values of the kinetic constants were qualitatively compared with the initial formation rates of campholenic aldehyde and trans-carveol showing similar trends and explaining well the prominent solvent effect of a basic solvent favoring formation of trans-carveol.
Abbreviations
- c :
-
Concentration (mol/l)
- E A :
-
Activation energy (kJ/mol)
- EN T :
-
Solvent polarity
- K 0 j :
-
Equilibrium constant at standard conditions for reaction j
- k :
-
Rate constant
- n :
-
Moles (mol)
- P :
-
Pressure (bar)
- P 0 :
-
Standard pressure (bar)
- r 0 :
-
Initial rate (mmol/min/gcat)
- R :
-
Ideal gas constant (J/K/mol)
- R 2 :
-
Degree of explanation
- r :
-
Reaction rate
- T :
-
Absolute temperature (K)
- T 0 :
-
Absolute standard temperature (K)
- t:
-
Time (s)
- \(\widehat {y}\) :
-
Estimate of concentration
- ΔG 0 f :
-
Gibbs free energy of formation at standard conditions (J/mol)
- ΔG 0 r :
-
Gibbs free energy of reaction at standard conditions (J/mol)
- ΔG Φ r,j :
-
Gibbs free energy of reaction at 1 bar and a chosen temperature (J/mol)
- ΔH 0 f :
-
Enthalpy of formation at standard conditions (J/mol)
- ΔH 0 r :
-
Enthalpy of reaction at standard conditions (J/mol)
- \(\theta\) :
-
Objective function
- ν i,j :
-
Stoichiometric matrix composed by i components and j reactions
References
Wilson K, Renson A, Clark JH (1999) Catal Lett 61:51–55
Da Silva KA, Hoehne JL, Gusevskaya EV (2008) Chem Eur J 14:6166–6172
Shcherban ND, Barakov RYu, Mäki-Arvela P, Sergienko SA, Bezverkhyy I, Eränen K, Murzin DYu (2018) Appl Catal A 560:236–247
Stekrova M, Kumar N, Diaz SF, Mäki-Arvela P, Murzin DYu (2015) Catal Today 241:237–245
Kumar N, Mäki-Arvela P, Faten Diaz S, Aho A, Demidova Y, Linden J, Shepidchenko A, Tenhu M, Salonen J, Laukkanen P, Lashkul A, Dahl J, Sinev I, Leino AR, Kordas K, Salmi T, Murzin DYu (2013) Top Catal 56:696–713
Stekrova M, Kumar N, Aho A, Sinev I, Grunert W, Dahl J, Roine J, Arzumanov SS, Mäki-Arvela P, Murzin DYu (2014) Appl Catal A 470:162–196
Stekrova M, Kumar N, Mäki-Arvela P, Ardashov O, Volcho K, Salakhutdinov N, Murzin D (2013) Materials 6:2103–2118
Stekrova M, Kubů M, Shamzhy M, Musilová Z, Čejka J (2018) Catal Sci Technol 8:2488–2501
Vicevic M, Boodhoo KVK, Scott K (2007) Chem Eng J 133:31–41
Fahlbusch KG, Hammerschmidt FJ, Panten J, Pickenhagen W, Schatlowski D, Bauer K, Garbe D, Surberg H (2005) Flavors and fragrances in Ullman’s encyclopedia of industrial chemistry. Wiley, Weinheim
Bertero NM, Trasarti AF, Apesteguia CR, Marchi AJ (2011) Appl Catal A Gen 394:228–238
Sidorenko AYu, Ignatovich ZhV, Ermolinskaya AL, Kravtsova AV, Baranovskii AV, Koroleva EV, Agabekov VE (2018) Chem Nat Compd 54(5):893–897
Joback KG (1984) A Unified Approach to Physical Property Estimation Using Multivariate Statistical Techniques, Thesis SM, Department of Chemical Engineering, Massachusetts Institute of Technology, Cambridge
Joback KG, Reid RC (1987) Chem Eng Commun 57:233–243
Zemansky MW, Abbott MM, Van Ness HC (1975) Basic engineering thermodynamics. McGraw-Hill, New York
Poling BE, Prausnitz JM, O’Connell JP (2004) The properties of gases and liquids, 5th edn. McGraw-Hill, New York
International zeolite association, http://www.iza-structure.org/databases/. Accessed 25 Oct 2018
Reichardt C (1994) Chem Rev 94:2319–2358
Haario H (2007) Modest users guide. Profmath Oy, Helsinki
Acknowledgments
Open access funding provided by Abo Akademi University (ABO).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mäki-Arvela, P., Shcherban, N., Lozachmeur, C. et al. Isomerization of α-Pinene Oxide: Solvent Effects, Kinetics and Thermodynamics. Catal Lett 149, 203–214 (2019). https://doi.org/10.1007/s10562-018-2617-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-018-2617-8