Abstract
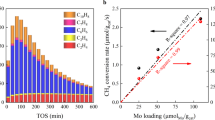
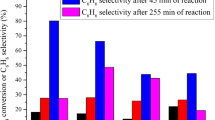
We present a new class of catalysts, InMo-ZSM-5, which can be prepared by indium impregnation of Mo-ZSM-5. The incorporation of indium dramatically decreases coke formation during methane dehydroaromatization. The benzene and C2 hydrocarbons selectivity among total hydrocarbons over InMo-ZSM-5 remains comparable to that of Mo-ZSM-5 despite reduced methane conversion due to decreased coke formation. We found 1 wt% indium to be optimal loading for reducing coke selectivity to half that of Mo-ZSM-5. Characterization methods were not helpful in discerning the interaction of In with Mo but experiments with bimetallic 1In2Mo-ZSM-5 and mechanical mixture 1In+2Mo-ZSM-5 suggest that In and Mo need to be in close proximity to suppress coke formation. This is supported by temperature programmed reduction experiments which show that In incorporation leads to lower Mo reduction temperature in In2Mo-ZMS-5.
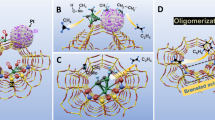
Graphical Abstract








Similar content being viewed by others
References
BP Statistical Review of World Energy 2013. Document retrieved from: http://www.bp.com on 16th of October, 2014.
Rostrup-Nielsen JR (1993) Catal Today 18:305–324
Spivey J, Hutchings G (2014) Chem Soc Rev 43:792–803 (and references therein)
Wang L, Tao L, Xie M, Xu G, Huang J, Xu Y (1993) Catal Lett 21:35–41
Majhi S, Mohanty P, Wang H, Pant KK (2013) J. Energy Chem 22:543–554 (and references therein)
Ismagilove Z, Matus E, Tsikoza L (2008) Energy Environ Sci 1:526–541 (and references therein)
Zeng J-L, Xiong Z-T, Zhang H-B, Lin G-D, Tsai KR (1998) Catal Lett 53:119–124
Weckhuysen BM, Wang D, Rosynek MP, Lunsford JH (1998) J Catal 175:338–346
Wang H, Liu Z, Shen J, Liu H, Zhangn J (2005) Catal Commmun 6:343–346
Lezcano-Gonzalez I, Oord R, Rovezzi M, Glatzel P, Botchway SW, Weckhuysen BM, Beale, AM (2016) Angew Chem Inter Ed 55:5215–5219
Tempelman CHL, Hensen EJM (2015) App Cata B 176: 731–739
Xu YB, Wang JD, Suzuki Y, Zhang ZG (2012) Catal Today 185:41–46
Liu S, Dong Q, Ohnishi R, Ichikawa M (1997) Chem Commun 1455–1456
Chen L, Lin L, Xu Z, Zhang T, Li X (1996) Catal Lett 39:169–172
Kojima R, Kikuchi S, Ma H, Bai J, Ichikawa M (2006) Catal Lett 110:15–21
Shu Y, Xu Y, Wong S-T, Wang L, Guo X (1997) J Catal 170:11–19
Liu B, Yang Y, Sayari A (2001) Appl Catal A 214:95–102
Al-Dughaither AS, de Lasa H (2014) Ind Eng Chem Res 53:15303–15316
Liu Q, Lu W, Tang J, Lin J, Fang JY (2005) J Am Chem Soc 127:5276–5277
O’Brien MG, Beale AM, Jacques SDM, Buslabs T, Honimaki V, Weckhuysen BM (2009) J Phys Chem C 113:4890–4897
Xu Y, Shu Y, Liu S, Huang J, Guo X (1995) Catal Lett 35:233–243
Williams CC, Ekerdt JG, Jehng J-M, Hardcastle FD, Turek AM, Wachs IE (1991) J Phys Chem 95:8781–8791
Berengue OM, Rodrigues AD, Dalmaschio CJ, Lanfredi AJC, Leite ER, Chiquito AJ (2010) J Phys D: Appl Phys 43:040501
Du J, Yang M, Cha SN, Rhen D, Kang M, Kang DJ (2008) Cryst Growth Des 8:2312–2317
Yang X, Wu Z, Moses-Debusk M, Mullins DR, Mahurin SM, Geiger RA, Kidder M, Narula CK (2012) J Phys Chem C 116:23322–23331
Borry III RW, Lu EC, Kim YH, Iglesia E (1997) Non-oxidative conversion of methane with continuous hydrogen removal, US Dept. of Energy/NETL, Morgantown, WV, Contract DE-AC03–76SF00098
Jiang H, Wang L, Cui W, Xu Y (1999) Catal Lett 57:95–102
Mihalyi RM, Schay Z, Szegedi A (2009) Catal Today 143:253–260
Acknowledgments
This research is sponsored by the Laboratory Directed Research and Development Program of Oak Ridge National Laboratory, managed by UT-Battelle, LLC, for the US Department of Energy. Authors thank Andrew Lepore for critical reading of manuscript. We also thank Shreya Celly, a summer undergraduate intern, for assistance with some of the experiments. Raman microscopy work is supported by Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the US Department of Energy. TPR work was supported by US Department of Energy, Office of Science, Basic Energy of Science, Chemical Science, Geoscience and Bioscience Division.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Kidder, M., Ruther, R.E. et al. Promotional Effects of In on Non-Oxidative Methane Transformation Over Mo-ZSM-5. Catal Lett 146, 1903–1909 (2016). https://doi.org/10.1007/s10562-016-1831-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-016-1831-5