Abstract
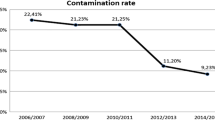
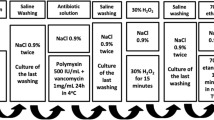
As a consequence of the preference for homologous tissues, bone banks are the primary source of bone and tendon grafts. However, the bacterial, viral, and fungal contamination of these grafts remains a considerable challenge in bone banks and often results in high rates of graft discarding and infections in patients. This study intended to investigate bacterial contamination in 509 bone grafts harvested from 110 multiorgan donors. Specimen collection included bone and soft tissue retrieved from culture-swabbing as well as bone and capsule for histopathology. Microbiological, histopathological, and radiographic analyses were carried out. Secondary sterilization was also conducted using cobalt 60 at the dose of 2.5 × 104 Gy. There were 106 multi-organ donors. Of the 506 grafts, there were 54 Hemi pelvis, 191 femur, 142 tibia, and 119 fibulae. The surface swab contamination rate for all the grafts retrieved was 16.6%, and bone culture from all the grafts was 6.1%. When we looked at the incidence of contamination according to the location than the surface swab contamination rate for hemipelvis was 18 (33.3%), femur 30 (15.7%), tibia 21(14.7%) and fibula 15 (12.6%). The bone cultures were hemipelvis 12 (22.2%) femur 8 (4.1%), tibia 5 (3.5%) and fibula 6 (5.04%). These findings suggest that separate harvesting of the grafts in reverse order may help prevent contamination. The study also recommends discarding all grafts contaminated even with low pathogenicity organisms. However, bioburden needs to be further investigated to be detected and reduced.
Similar content being viewed by others
References
Al-Mohrej OA, Al-Dera H (2015) Physiological principles that promote bone regeneration and repair during the application of ilizarov procedure. Sci Adv Mater 7:2134–2146. https://doi.org/10.1166/sam.2015.2639
Chahbouni M, Rami A, El Morhit M et al (2018) Creation of the bone bank of the rabat and casablanca region in Morocco. Pan Afr Med J 29. https://doi.org/10.11604/pamj.2018.29.210.14343
Daly PJ, Fitzgerald RH, Melton LJ, Llstrup DM (1987) Epidemiology of ankle fractures in Rochester, Minnesota. Acta Orthop 58:539–544. https://doi.org/10.3109/17453678709146395
Deijkers RLM, Bloem RM, Petit PLC et al (1997) Contamination of bone allografts. J Bone Jt Surg B 79:161–166. https://doi.org/10.1302/0301-620X.79B1.7137
Fitzgerald RH, Peterson LFA, Washington JA et al (1973) Bacterial colonization of wounds and sepsis in total hip arthroplasty. J Bone Jt Surg A 55:1242–1250. https://doi.org/10.2106/00004623-197355060-00011
Friedlaender GE (1987) Bone banking: In support of reconstructive surgery of the hip. Clin Orthop Relat Res, 17–21. https://doi.org/10.1097/00003086-198712000-00004
Giannoudis PV, Dinopoulos H, Tsiridis E (2005) Bone substitutes: an update. Injury 36(Suppl):3
Greenwald MA, Kuehnert MJ, Fishman JA (2012) Infectious disease transmission during organ and tissue transplantation. Emerg Infect Dis 18. https://doi.org/10.3201/eid1808.120277
Ibrahim T, Stafford H, Esler CNA, Power RA (2004) Cadaveric allograft microbiology. Int Orthop 28:315–318. https://doi.org/10.1007/s00264-004-0579-5
Ilyas I, Al-Mohrej OA (2020) High incidence of irradiated cortical strut allograft resorption following revision of femoral stems. J Arthroplasty. https://doi.org/10.1016/j.arth.2020.10.023
Ilyas I, Alrumaih H, Rabbani S (2017) Freeze dried proximal femoral allografts in revision of femoral stems. J Arthroplasty 32:171–176. https://doi.org/10.1016/j.arth.2016.06.016
Ilyas I, Al-Rabiah AM, Alhussainan TS et al (2020) Principles of bone and tissue banking in Saudi Arabia: 10-year experience report. Cell Tissue Bank. https://doi.org/10.1007/s10561-020-09868-7
Journeaux SF, Johnson N, Bryce SL et al (1999) Bacterial contamination rates during bone allograft retrieval. J Arthroplasty 14:677–681. https://doi.org/10.1016/S0883-5403(99)90222-X
Judas F, Teixeira L, Proença A (2005) Coimbra University Hospitals’ bone and tissue bank: twenty-two years of experience. Transplant Proc. https://doi.org/10.1016/j.transproceed.2005.05.004
Khan MT, Stockley I, Ibbotson C (1998) Allograft bone transplantation: A Sheffield experience. Ann R Coll Surg Engl
Kurup HV, Rao P, Patro DK (2004) Bone allografting: an Indian experience. Int Orthop. https://doi.org/10.1007/s00264-004-0593-7
Lord CF, Gebhardt MC, Tomford WW, Mankin HJ (1988) Infection in bone allografts. Incidence, nature, and treatment. J Bone Jt Surg A 70:369–376. https://doi.org/10.2106/00004623-198870030-00008
Malinin TI, Martinez OV, Brown MD (1985) Banking of massive osteoarticular and intercalary bone allografts—12 years’ experience. Clin Orthop Relat Res NO 197:44–57. https://doi.org/10.1097/00003086-198507000-00007
Mankin HJ, Gebhardt MC, Jennings LC et al (1996) Long-term results of allograft replacement in the management of bone tumors. Clin Orthop Relat Res, 86–97. https://doi.org/10.1097/00003086-199603000-00011
Nather A (2004) Musculoskeletal tissue banking in Singapore: 15 years of experience (1988–2003). J Orthop Surg (Hong Kong) 12:184–190. https://doi.org/10.1177/230949900401200209
Nguyen H, Morgan DAF, Forwood MR (2007) Sterilization of allograft bone: is 25 kGy the gold standard for gamma irradiation? Cell Tissue Bank 8:81–91. https://doi.org/10.1007/s10561-006-9019-7
Panegrossi G, Ceretti M, Papalia M et al (2014) Bone loss management in total knee revision surgery. Int Orthop 38:419–427. https://doi.org/10.1007/s00264-013-2262-1
Raizman NM, O’brien JR, Poehling-Monaghan KL, Yu WD (2009) Pseudarthrosis of the spine. J Am Acad Orthop Surg 17:494–503
Shegarfi H, Reikeras O (2009) Review article: bone transplantation and immune response. J Orthop Surg (Hong Kong) 17:206–211. https://doi.org/10.1177/230949900901700218
Simonds RJ, Holmberg SD, Coleman TR et al (1992) Transmission of human immunodeficiency virus type 1 from a seronegative organ and tissue donor. N Engl J Med 326:726–732. https://doi.org/10.1056/NEJM199203123261102
Slooff TJJH, Buma P, Schreurs BW et al (1996) Acetabular and femoral reconstruction with impacted graft and cement. Clin Orthopaedics Related Res, pp 108–115
Tomford WW, Starkweather RJ, Goldman MH (1981) A study of the clinical incidence of infection in the use of banked allograft bone. J Bone Jt Surg A 63:244–248. https://doi.org/10.2106/00004623-198163020-00010
Tomford WW, Mankin HJ, Friedlaender GE et al (1987) Methods of banking bone and cartilage for allograft transplantation. Orthop Clin North Am 18:241–247
Vajaradul Y (2000) Bangkok biomaterial center: 15 years experience in tissue banking. Cell Tissue Bank 1:229–239. https://doi.org/10.1023/A:1026542730541
Acknowledgements
Words seem inadequate to express our sadness over ABF who passed away during the peer-reviewing process. He was, is now, and will remain in our thoughts and prayers.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have declared that no competing interests exist.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The present study was approved by the Institutional Review Board (IRB) at KFSH&RC, Riyadh, Saudi Arabia.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ilays, I., Alsakran, S.A., Fallatah, A.B. et al. The contamination of allografts in multi-organ donors: a bone bank experience. Cell Tissue Bank 22, 499–504 (2021). https://doi.org/10.1007/s10561-020-09899-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-020-09899-0