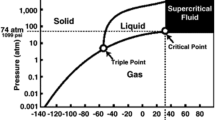
Abstract
Sterilisation of allografts are a crucial step in ensuring safety and viability. Current sterilisation standards such as 25 kGy gamma irradiation (γ) can have adverse effects on the ultrastructure and biomechanical properties of allograft tissue. Supercritical CO2 (SCCO2) technology, represents an improved sterilisation process that potentially preserves tissue properties. This study aimed to test the effect of SCCO2 sterilisation on the biomechanical and histological properties of the meniscus and compare this to the current standard of γ. Thirty-two 18-month old ovine menisci were randomly assigned into three groups for sterilisation (SCCO2, γ and control). After treatment, biomechanical indentation testing (stiffness and stress relaxation) or histological analysis [percentage of void, cells and extracellular matrix (ECM) per slide] was undertaken. Both SCCO2 and gamma groups displayed an increase in stiffness and stress relaxation as compared to control, however, this difference was lesser in samples treated with SCCO2. No significant histological quantitative differences were detected between SCCO2 and control specimens. Gamma-treated samples demonstrated a significant increase in void and decrease in ECM. Interestingly, both treatment groups demonstrated a decreasing mean void and increasing ECM percentage when analysed from outer to inner zones. No significant differences were detected in all-endpoints when analysed by section. SCCO2 sterilisation represents a potential feasible alternative to existing sterilization techniques such as γ.








Similar content being viewed by others
References
Adams M, Hukins D (1992) The extracellular matrix of the meniscus. In: Mow VC, Arnoczky SP, Jackson DW (eds) Knee meniscus: basic and clinical foundations. Raven Press, New York
Akkus O, Belaney RM, Das P (2005) Free radical scavenging alleviates the biomechanical impairment of gamma radiation sterilized bone tissue. J Orthop Res 23:838–845. doi:10.1016/j.orthres.2005.01.007
Barry JJ, Silva MM, Popov VK, Shakesheff KM, Howdle SM (2006) Supercritical carbon dioxide: putting the fizz into biomaterials. Philos Trans Ser A Math Phys Eng Sci 364:249–261. doi:10.1098/rsta.2005.1687
Brindle T, Nyland J, Johnson DL (2001) The meniscus: review of basic principles with application to surgery and rehabilitation. J Athl Train 36:160–169
Brunner G (2005) Supercritical fluids: technology and application to food processing. J Food Eng 67:21–33. doi:10.1016/j.jfoodeng.2004.05.060
Campbell DG, Li P (1999) Sterilization of HIV with irradiation: relevance to infected bone allografts. Aust N Z J Surg 69:517–521
Cheung DT, Perelman N, Tong D, Nimni ME (1990) The effect of gamma-irradiation on collagen molecules, isolated alpha-chains, and crosslinked native fibers. J Biomed Mater Res 24:581–589. doi:10.1002/jbm.820240505
Chevrier A, Nelea M, Hurtig MB, Hoemann CD, Buschmann MD (2009) Meniscus structure in human, sheep, and rabbit for animal models of meniscus repair. J Orthop Res 27:1197–1203. doi:10.1002/jor.20869
Christensen TW, Burns D, White A, Ganem B, Eisenhunt A (2004) Sterilization methods and apparatus which employ additive-containing supercritical carbon dioxide sterilant. US 7108832 B2, 2006
Cinquemani C, Boyle C, Bach E, Schollmeyer E (2007) Inactivation of microbes using compressed carbon dioxide: an environmentally sound disinfection process for medical fabrics. J Supercrit Fluids 42:392–397. doi:10.1016/j.supflu.2006.11.001
Englund M, Guermazi A, Lohmander SL (2009) The role of the meniscus in knee osteoarthritis: a cause or consequence? Radiol Clin N Am 47:703–712. doi:10.1016/j.rcl.2009.03.003
Englund M, Roemer FW, Hayashi D, Crema MD, Guermazi A (2012) Meniscus pathology, osteoarthritis and the treatment controversy. Nat Rev Rheumatol 8:412–419. doi:10.1038/nrrheum.2012.69
Fages J et al (1998) Viral inactivation of human bone tissue using supercritical fluid extraction. ASAIO J 44:289–293
Fernandes JC, Martel-Pelletier J, Pelletier JP (2002) The role of cytokines in osteoarthritis pathophysiology. Biorheology 39:237–246
Furukawa S, Watanabe T, Koyama T, Hirata J, Narisawa N, Ogihara H, Yamasaki M (2009) Inactivation of food poisoning bacteria and Geobacillus stearothermophilus spores by high pressure carbon dioxide treatment. Food Control 20:53–58. doi:10.1016/j.foodcont.2008.02.002
Grieb TA et al (2006) High-dose gamma irradiation for soft tissue allografts: high margin of safety with biomechanical integrity. J Orthop Res 24:1011–1018. doi:10.1002/jor.20079
Harner CD, Lo MY (2009) Future of allografts in sports medicine. Clin Sports Med 28: 327–340, ix. doi:10.1016/j.csm.2008.10.010
Ireland L, Spelman D (2005) Bacterial contamination of tissue allografts: experiences of the donor tissue bank of Victoria. Cell Tissue Bank 6:181–189. doi:10.1007/s10561-005-7365-5
Kim SH, Jung Y, Kim SH (2013) A biocompatible tissue scaffold produced by supercritical fluid processing for cartilage tissue engineering. Tissue Eng Part C Methods 19:181–188
Lee AS, Kang RW, Kroin E, Verma NN, Cole BJ (2012) Allograft meniscus transplantation. Sports Med Arthrosc 20:106–114. doi:10.1097/Jsa.0b013e318246f005
Lewis PB, Williams JM, Hallab N, Virdi A, Yanke A, Cole BJ (2008) Multiple freeze-thaw cycled meniscal allograft tissue: a biomechanical, biochemical, and histologic analysis. J Orthop Res 26:49–55. doi:10.1002/jor.20473
Lubowitz JH, Verdonk PCM, Reid JB, Verdonk R (2007) Meniscus allograft transplantation: a current concepts review. Knee Surg Sports Traumatol Anthrosc 15:476–492. doi:10.1007/s00167-006-0216-5
Maier D et al (2007) In vitro analysis of an allogenic scaffold for tissue-engineered meniscus replacement. J Orthop Res 25:1598–1608. doi:10.1002/jor.20405
McAllister DR, Joyce MJ, Mann BJ, Vangsness CT Jr (2007) Allograft update: the current status of tissue regulation, procurement, processing, and sterilization. Am J Sports Med 35:2148–2158. doi:10.1177/0363546507308936
McNickle AG, Wang VM, Shewman EF, Cole BJ, Williams JM (2009) Performance of a sterile meniscal allograft in an ovine model. Clin Orthop Relat Res 467:1868–1876. doi:10.1007/s11999-008-0567-y
Melo Silva J, Rigo AA, Dalmolin IA, Debien I, Cansian RL, Oliveira JV, Mazutti MA (2013) Effect of pressure, depressurization rate and pressure cycling on the inactivation of Escherichia coli by supercritical carbon dioxide. Food Control 29:76–81. doi:10.1016/j.foodcont.2012.05.068
Mickiewicz P, Binkowski M, Bursig H, Wrobel Z (2014) Preservation and sterilization methods of the meniscal allografts: literature review. Cell Tissue Bank 15:307–317. doi:10.1007/s10561-013-9396-7
Mun S, Hahn JS, Lee YW, Yoon J (2011) Inactivation behavior of Pseudomonas aeruginosa by supercritical N2O compared to supercritical CO2. Int J Food Microbiol 144:372–378. doi:10.1016/j.ijfoodmicro.2010.10.022
Naal FD, Schauwecker J, Steinhauser E, Milz S, von Knoch F, Mittelmeier W, Diehl P (2008) Biomechanical and immunohistochemical properties of meniscal cartilage after high hydrostatic pressure treatment. J Biomed Mater Res B 87B:19–25. doi:10.1002/Jbm.B.31059
Nemzek JA, Arnoczky SP, Swenson CL (1994) Retroviral transmission by the transplantation of connective-tissue allografts. An experimental study. J Bone Joint Surg Am 76:1036–1041
Nichols A, Burns D, Christopher R (2009) Studies on the sterilization of human bone and tendon musculoskeletal allograft tissue using supercritical carbon dioxide. J Orthop. 6:9–17
Perrut M (2012) Sterilization and virus inactivation by supercritical fluids (a review). J Supercrit Fluids 66:359–371. doi:10.1016/j.supflu.2011.07.007
Proffen BL, McElfresh M, Fleming BC, Murray MM (2012) A comparative anatomical study of the human knee and six animal species. Knee 19:493–499. doi:10.1016/j.knee.2011.07.005
Qiu QQ, Leamy P, Brittingham J, Pomerleau J, Kabaria N, Connor J (2009) Inactivation of bacterial spores and viruses in biological material using supercritical carbon dioxide with sterilant. J Biomed Mater Res B Appl Biomater 91:572–578
Rijk PC (2004) Meniscal allograft transplantation: part I: background, results, graft selection and preservation, and surgical considerations. Arthroscopy 20:728–743. doi:10.1016/j.arthro.2004.06.015
Russell NA, Pelletier MH, Bruce WJ, Walsh WR (2012a) The effect of gamma irradiation on the anisotropy of bovine cortical bone. Med Eng Phys 34:1117–1122. doi:10.1016/j.medengphy.2011.11.021
Russell NA, Rives A, Pelletier MH, Bruce WJ, Walsh WR (2012b) The effect of sterilization on the mechanical properties of intact rabbit humeri in three-point bending, four-point bending and torsion. Cell Tissue Bank. doi:10.1007/s10561-012-9318-0
Sandmann GH et al (2009) Generation and characterization of a human acellular meniscus scaffold for tissue engineering. J Biomed Mater Res A 91:567–574. doi:10.1002/jbm.a.32269
Stone KR, Adelson WS, Pelsis JR, Walgenbach AW, Turek TJ (2010) Long-term survival of concurrent meniscus allograft transplantation and repair of the articular cartilage A PROSPECTIVE TWO- TO 12-YEAR FOLLOW-UP REPORT. J Bone Joint Surg Br 92B:941–948. doi:10.1302/0301-620x.92b7.23182
Tarafa PJ, Jiménez A, Zhang J, Matthews MA (2010) Compressed carbon dioxide (CO2) for decontamination of biomaterials and tissue scaffolds. J Supercrit Fluids 53:192–199
Temtem M et al (2009) Supercritical CO2 generating chitosan devices with controlled morphology. Potential application for drug delivery and mesenchymal stem cell culture. J Supercrit Fluids 48:269–277. doi:10.1016/j.supflu.2008.10.020
TGA (2011) Regulatory life cycle for biologicals that are included on the Australian Register of Therapeutic Goods vol 1.0, 1 edn. Australian Government, Canberra, Australia
Vaishnav S, Thomas Vangsness C Jr, Dellamaggiora R (2009) New techniques in allograft tissue processing. Clin Sports Med 28:127–141
Vangsness CT, Garcia IA, Mills CR, Kainer MA, Roberts MR, Moore TM (2003) Allograft transplantation in the knee: tissue regulation, procurement, processing, and sterilization. Am J Sports Med 31:474–481
Vangsness CT, Jr, Dellamaggiora RD (2009) Current safety sterilization and tissue banking issues for soft tissue allografts. Clin Sports Med. 28:183–189, vii doi:10.1016/j.csm.2008.10.008
Verdonk PCM, Demurie A, Almqvist F, Veys EM, Verbruggen G, Verdonk R (2005) Transplantation of viable meniscal allograft: survivorship analysis and clinical outcome of one hundred cases. J Bone Joint Surg Am 87A:715–724. doi:10.2106/Jbjs.C.01344
White A, Burns D, Christensen TW (2006) Effective terminal sterilization using supercritical carbon dioxide. J Biotechnol 123:504–515
Wilusz RE, Weinberg JB, Guilak F, McNulty AL (2008) Inhibition of integrative repair of the meniscus following acute exposure to interleukin-1 in vitro. J Orthop Res 26:504–512. doi:10.1002/jor.20538
Zhang J, Davis TA, Matthews MA, Drews MJ, LaBerge M, An YH (2006) Sterilization using high-pressure carbon dioxide. J Supercrit Fluids 38:354–372. doi:10.1016/j.supflu.2005.05.005
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bui, D., Lovric, V., Oliver, R. et al. Meniscal allograft sterilisation: effect on biomechanical and histological properties. Cell Tissue Bank 16, 467–475 (2015). https://doi.org/10.1007/s10561-014-9492-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-014-9492-3