Abstract
Background
Cardiac hypertrophy is the common pathological process of multiple cardiovascular diseases. However, the molecular mechanisms of cardiac hypertrophy are unclear. Long non-coding RNA (lncRNA), a newly discovered type of transcript that has been demonstrated to function as crucial regulators in the development of cardiovascular diseases. This study revealed a novel regulatory pathway of lncRNA in cardiac hypertrophy.
Methods
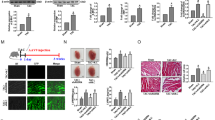
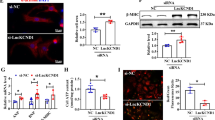
The cardiac hypertrophy models were established by transverse aortic constriction (TAC) in mice and angiotensin II (Ang II) in HL-1 cardiomyocytes. Adeno-associated virus 9 (AAV9) in vivo and lncRNA Gm15834 and shRNA plasmids in vitro were used to overexpress and knock down lncRNA Gm15834. The myocardial tissue structure, cardiomyocyte area, cardiac function, protein expressions, and binding of lncRNA Gm15834 and Src-associated substrate during mitosis of 68 KDa (Sam68) were detected by hematoxylin and eosin (HE) staining, immunofluorescence staining, echocardiography, western blot and RNA immunoprecipitation (RIP), respectively.
Results
In cardiac hypertrophy models, inhibiting lncRNA Gm15834 could decrease Sam68 expression and nuclear factor kappa-B (NF-κB) mediated inflammatory activities in vivo and in vitro, but overexpressing lncRNA Gm15834 showed the opposite results. RIP experiments validated the binding activities between lncRNA Gm15834 and Sam68. Overexpression of Sam68 could counteract the anti-hypertrophy effects of lncRNA Gm15834 knockdown. Meanwhile, in vivo inhibition of lncRNA Gm15834 could inhibit Sam68 expression, reduce NF-κB mediated inflammatory activity and attenuate cardiac hypertrophy.
Conclusion
Our study revealed a novel regulatory axis of cardiac hypertrophy, which comprised lncRNA Gm15834/Sam68/NF-κB/inflammation, shedding a new light for identifying therapy target of cardiac hypertrophy in clinic.








Similar content being viewed by others
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Heron M, Anderson RN. Changes in the leading cause of death: recent patterns in heart disease and cancer mortality. NCHS Data Brief. 2016;(254):1–8.
Sabbah HN. Silent disease progression in clinically stable heart failure. Eur J Heart Fail. 2017;19:469–78. https://doi.org/10.1002/ejhf.705.
Xiong Z, Sun G, Zhu C, et al. Artemisinin, an anti-malarial agent, inhibits rat cardiac hypertrophy via inhibition of NF-κB signaling. Eur J Pharmacol. 2010;649:277–84. https://doi.org/10.1016/j.ejphar.2010.09.018.
Ma Z, Qin X, Zhong X, et al. Flavine adenine dinucleotide inhibits pathological cardiac hypertrophy and fibrosis through activating short chain acyl-CoA dehydrogenase. Biochem Pharmacol. 2020;178:114100. https://doi.org/10.1016/j.bcp.2020.114100.
Raut GK, Manchineela S, Chakrabarti M, et al. Imine stilbene analog ameliorate isoproterenol-induced cardiac hypertrophy and hydrogen peroxide-induced apoptosis. Free Radic Biol Med. 2020;153:80–8. https://doi.org/10.1016/j.freeradbiomed.2020.04.014.
Spizzo R, Almeida MI, Colombatti A, Calin GA. Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene. 2011;31:4577–87. https://doi.org/10.1038/onc.2011.621.
Palazzo AF, Lee ES. Non-coding RNA: what is functional and what is junk? Front Genet. 2015;6:2. https://doi.org/10.3389/fgene.2015.00002.
Adelman K, Egan E. Non-coding RNA: more uses for genomic junk. Nature. 2017;543:183–5. https://doi.org/10.1038/543183a.
Uchida S, Dimmeler S. Long noncoding RNAs in cardiovascular diseases. Circ Res. 2015;116:737–50. https://doi.org/10.1161/CIRCRESAHA.116.302521.
Bär C, Chatterjee S, Thum T. Long noncoding RNAs in cardiovascular pathology, diagnosis, and therapy. Circulation. 2016;134:1484–99. https://doi.org/10.1161/CIRCULATIONAHA.116.023686.
Wang Z, Zhang XJ, Ji YX, et al. The long noncoding RNA chaer defines an epigenetic checkpoint in cardiac hypertrophy. Nat Med. 2016;22:1131–9. https://doi.org/10.1038/nm.4179.
Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–9. https://doi.org/10.1038/nrg2521.
Chen J, Zhang J, Gao Y, et al. LncSEA: a platform for long non-coding RNA related sets and enrichment analysis. Nucleic Acids Res. 2021;49:D969–d980. https://doi.org/10.1093/nar/gkaa806.
Liu CY, Zhang YH, Li RB, et al. LncRNA CAIF inhibits autophagy and attenuates myocardial infarction by blocking p53-mediated myocardin transcription. Nat Commun. 2018;9:29. https://doi.org/10.1038/s41467-017-02280-y.
Zhang Y, Jiao L, Sun L, et al. LncRNA ZFAS1 as a SERCA2a inhibitor to cause intracellular Ca2+ overload and contractile dysfunction in a mouse model of myocardial infarction. Circ Res. 2018;122:1354–68. https://doi.org/10.1161/CIRCRESAHA.117.312117.
Song C, Qi H, Liu Y, et al. Inhibition of lncRNA Gm15834 attenuates autophagy-mediated myocardial hypertrophy via the miR-30b-3p/ ULK1 axis in mice. Mol Ther. 2021;29:1120–37. https://doi.org/10.1016/j.ymthe.2020.10.024.
Barlat I, Maurier F, Duchesne M, Guitard E, Tocque B, Schweighoffer F. A role for Sam68 in cell cycle progression antagonized by a spliced variant within the KH domain. J Biol Chem. 1997;272:3129–32. https://doi.org/10.1074/jbc.272.6.3129.
Paronetto MP, Achsel T, Massiello A, Chalfant CE, Sette C. The RNA-binding protein Sam68 modulates the alternative splicing of Bcl-x. J Cell Biol. 2007;176:929–39. https://doi.org/10.1083/jcb.200701005.
Najib S, Mart¨ªn-Romero C, Gonz¨¢lez-Yanes C, S¨¢nchez-Margalet V. Role of Sam68 as an adaptor protein in signal transduction. Cell Mol Life Sci. 2005;62:36–43. https://doi.org/10.1007/s00018-004-4309-3.
Ramakrishnan P, Baltimore D. Sam68 is required for both NF-κB activation and apoptosis signaling by the TNF receptor. Mol Cell. 2011;43:167–79. https://doi.org/10.1016/j.molcel.2011.05.007.
Fu K, Sun X, Wier EM, et al. Sam68/KHDRBS1 is critical for colon tumorigenesis by regulating genotoxic stress-induced NF-κB activation. Elife. 2016;5:e15018. https://doi.org/10.7554/eLife.15018.
Hall G, Hasday JD, Rogers TB. Regulating the regulator: NF-kappaB signaling in heart. J Mol Cell Cardiol. 2006;41. https://doi.org/10.1016/j.yjmcc.2006.07.006.:580 – 91.
Castello A, Fischer B, Hentze MW, Preiss T. RNA-binding proteins in mendelian disease. Trends Genet. 2013;29:318–27. https://doi.org/10.1016/j.tig.2013.01.004.
Chen CY, Shyu AB. Emerging mechanisms of mRNP remodeling regulation. Wiley Interdiscip Rev RNA. 2014;5:713–22. https://doi.org/10.1002/wrna.1241.
Luo X, He S, Hu Y, Liu J, Chen X. Sp1-induced LncRNA CTBP1-AS2 is a novel regulator in cardiomyocyte hypertrophy by interacting with FUS to stabilize TLR4. Cardiovasc Pathol. 2019;42:21–9. https://doi.org/10.1016/j.carpath.2019.04.005.
Chen C, Liu M, Tang Y, et al. LncRNA H19 is involved in myocardial ischemic preconditioning via increasing the stability of nucleol in protein. J Cell Physiol. 2020;235:5985–94. https://doi.org/10.1002/jcp.29524.
Xu Z, Mo Y, Li X, et al. The novel LncRNA AK035396 drives cardiomyocyte apoptosis through Mterf1 in myocardial ischemia/reperfusion injury. Front Cell Dev Biol. 2021;9:773381. https://doi.org/10.3389/fcell.2021.773381.
Mart¨ªn-Romero C, S¨¢nchez-Margalet V. Human leptin activates PI3K and MAPK pathways in human peripheral blood mononuclear cells: possible role of Sam68. Cell Immunol. 2001;212:83–91. https://doi.org/10.1006/cimm.2001.1851.
Kunkel GT, Wang X. Sam68 guest STARs in TNF-α signaling. Mol Cell. 2011;43(2):157–8. https://doi.org/10.1016/j.molcel.2011.07.004.
Tomalka JA, de Jesus TJ, Ramakrishnan P. Sam68 is a regulator of toll-like receptor signaling. Cell Mol Immunol. 2017;14:107–17. https://doi.org/10.1038/cmi.2016.32.
Zelarayan L, Renger A, Noack C, et al. NF-kappaB activation is required for adaptive cardiac hypertrophy. Cardiovasc Res. 2009;84:416–24. https://doi.org/10.1093/cvr/cvp237.
Hong HQ, Lu J, Fang XL, et al. G3BP2 is involved in isoproterenol-induced cardiac hypertrophy through activating the NF-κB signaling pathway. Acta Pharmacol Sin. 2018;39:184–94. https://doi.org/10.1038/aps.2017.58.
Wang GJ, Wang HX, Yao YS, Guo LY, Liu P. The role of Ca2+/calmodulin-dependent protein kinase II and calcineurin in TNF-α-induced myocardial hypertrophy. Braz J Med Biol Res. 2012;45:1045–51. https://doi.org/10.1590/s0100-879x2012007500121.
Zhang S, Tang F, Yang Y, et al. Astragaloside IV protects against isoproterenol-induced cardiac hypertrophy by regulating NF-κB/PGC-1α signaling mediated energy biosynthesis. PLoS ONE. 2015;10:e0118759. https://doi.org/10.1371/journal.pone.0118759.
Kumar S, Wang G, Zheng N, et al. HIMF (hypoxia-induced mitogenic factor)-IL (interleukin)-6 signaling mediates cardiomyocyte-fibroblast crosstalk to promote cardiac hypertrophy and fibrosis. Hypertension. 2019;73:1058–70. https://doi.org/10.1161/HYPERTENSIONAHA.118.12267.
Bai Y, Sun X, Chu Q, et al. Caspase-1 regulate AngII-induced cardiomyocyte hypertrophy via upregulation of IL-1β. Biosci Rep. 2018. https://doi.org/10.1042/BSR20171438. BSR20171438.
Funding
This study was supported by Key Project of Natural Science Foundation of Heilongjiang Province of China (ZD2017015).
Author information
Authors and Affiliations
Contributions
Hongli Sun and Chao Song participated in the conception and design of the study. Material preparation, data collection and analysis were performed by Yongsheng Liu, Man Jiang, Meitian Zhang, Yawen Xie, Lixin Wang, Pilong Shi, Qianlong Zhang, Qianhui Zhang, Kai Liu and Jiajun Zhou. The first draft of the manuscript was written by Yongsheng Liu. All authors have commented on previous versions of the manuscript. All authors have read and approved the final draft.
Corresponding authors
Ethics declarations
Ethics Approval
The present study was conducted according to the “Guide for the Care and Use of Laboratory Animals” (NIH publication, 8th Edition, 2011). All animal experiments involved in this subject were approved and agreed by the Ethics Committee of Harbin Medical University-Daqing.
Consent to Participate
Not applicable.
Consent for Publication
Authors give full consent for publication.
Competing Interests
The authors declare that they have no conflicts of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Jiang, M., Zhang, M. et al. LncRNA Gm15834 Aggravates Cardiac Hypertrophy by Interacting with Sam68 and Activating NF-κB Mediated Inflammation. Cardiovasc Drugs Ther (2024). https://doi.org/10.1007/s10557-024-07569-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s10557-024-07569-x