Abstract
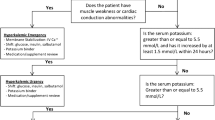
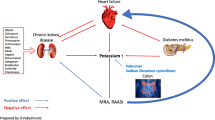
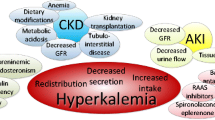
Hyperkalaemia is one of the most common electrolyte disorders in patients with cardiovascular disease (CVD). The true burden of hyperkalaemia in the real-world setting can be difficult to assess, but in population-based cohort studies up to 4 in 10 patients developed hyperkalaemia. In addition to drugs interfering with potassium metabolism and food intake, several conditions can cause or worsen hyperkalaemia, such as advanced age, diabetes, and chronic kidney disease. Mortality, cardiovascular morbidity, and hospitalisation are higher in patients with hyperkalaemia. Hyperkalaemia represents a major contraindication or a withholding cause for disease-modifying therapies like renin–angiotensin–aldosterone inhibitors (RAASi), mainly mineralocorticoid receptor antagonists. Hyperkalaemia can be also classified as acute and chronic, according to the onset. Acute hyperkalaemia is often a life-threatening emergency requiring immediate treatment to avoid lethal arrhythmias. Therapy goal is cell membrane stabilisation by calcium administration, cellular intake, shift of extracellular potassium to the intracellular space (insulin, beta-adrenergic agents, sodium bicarbonate), and increased elimination with diuretics or dialysis. Chronic hyperkalaemia was often managed with dietary counselling to prevent potassium-rich food intake and tapering of potassium-increasing drugs, mostly RAASi. Sodium polystyrene sulphonate, a potassium binder, was the only therapeutic option. Recently, new drugs such as patiromer and sodium zirconium cyclosilicate give new opportunities for the treatment of hyperkalaemia, as they proved to be safe, well tolerated, and effective. Aim of this review is to describe the burden of hyperkalaemia in cardiovascular patients, its direct and indirect effects, and the therapeutic options now available in the acute and chronic setting.


Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Thomsen RW, Nicolaisen SK, Hasvold P, et al. Elevated potassium levels in patients with congestive heart failure: occurrence, risk factors, and clinical outcomes: a Danish population-based cohort study. J Am Heart Assoc. 2018;7(11): e008912. https://doi.org/10.1161/JAHA.118.008912.
Collins AJ, Pitt B, Reaven N, et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46(3):213–21. https://doi.org/10.1159/000479802.
Núñez J, Bayés-Genís A, Zannad F, et al. Long-term potassium monitoring and dynamics in heart failure and risk of mortality. Circulation. 2018;137(13):1320–30. https://doi.org/10.1161/CIRCULATIONAHA.117.030576.
Ferreira JP, Rossignol P, Machu JL, et al. Mineralocorticoid receptor antagonist pattern of use in heart failure with reduced ejection fraction: findings from BIOSTAT-CHF. Eur J Heart Fail. 2017;19:1284–93. https://doi.org/10.1002/ejhf.90.
Savarese G, Carrero JJ, Pitt B, et al. Factors associated with underuse of mineralocorticoid receptor antagonists in heart failure with reduced ejection fraction: an analysis of 11 215 patients from the Swedish Heart Failure Registry. Eur J Heart Fail. 2018;20:1326–34. https://doi.org/10.1002/ejhf.1182.
Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Managed Care. 2015;21:S212–20.
Rosano GMC, Tamargo J, Kjeldsen KP, et al. Expert consensus document on the management of hyperkalaemia in patients with cardiovascular disease treated with renin angiotensin aldosterone system inhibitors: coordinated by the Working Group on Cardiovascular Pharmacotherapy of the European Society of Cardiology. European Hear J - Cardiovasc Pharmacother. 2018;4:180–8. https://doi.org/10.1093/ehjcvp/pvy015.
McLean RM, Wang NX. Potassium. Adv Food Nutr Res. 2021;96:89–121. https://doi.org/10.1016/bs.afnr.2021.02.013.
Unwin RJ, Luft FC, Shirley DG. Pathophysiology and management of hypokalemia: a clinical perspective. Nat Rev Nephrol. 2011;7:75–84. https://doi.org/10.1038/nrneph.2010.175.
Palmer BF, Carrero JJ, Clegg DJ, et al. Clinical management of hyperkalemia. Mayo Clin Proc. 2021;96:744–62. https://doi.org/10.1016/j.mayocp.2020.06.014.
Ewart HS, Klip A. Hormonal regulation of the Na(+)-K(+)-ATPase: mechanisms underlying rapid and sustained changes in pump activity. Am J Physiol. 1995;269(2 Pt 1):C295-311. https://doi.org/10.1152/ajpcell.1995.269.2.C295.
Allon M, Shanklin N. Adrenergic modulation of extrarenal potassium disposal in men with end-stage renal disease. Kidney Int. 1991;40:1103–9. https://doi.org/10.1038/ki.1991.321.
Mihailidou AS, Buhagiar KA, Rasmussen HH. Na+ influx and Na(+)-K+ pump activation during short-term exposure of cardiac myocytes to aldosterone. Am J Physiol. 1998;274:C175–81. https://doi.org/10.1152/ajpcell.1998.274.1.C175.
McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail. 2022;24:4–131. https://doi.org/10.1093/eurheartj/ehab368.
Ranjitkar P, Greene DN, Baird GS, Hoofnagle AN, Mathias PC. Establishing evidence-based thresholds and laboratory practices to reduce inappropriate treatment of pseudohyperkalemia. Clin Biochem. 2017;50:663–9. https://doi.org/10.1016/j.clinbiochem.2017.03.007.
Roccaforte V, Daves M, Alfreijat A, et al. Spurious elevation of serum potassium concentration measured in samples with thrombocytosis. Diagnosis (Berl). 2016;3:71–4. https://doi.org/10.1515/dx-2016-0008.
Liu S, Zhang L, Weng H, Yang F, Jin H, Fan F, Zheng X, Yang H, Li H, Zhang Y, Li J. Association between average plasma potassium levels and 30-day mortality during hospitalization in patients with COVID-19 in Wuhan. China Int J Med Sci. 2021Jan 1;18(3):736–43. https://doi.org/10.7150/ijms.50965.
Pannall P, Rossi A. Potassium levels in serum and plasma. Clin Chim Acta. 1970;30(1):218–20. https://doi.org/10.1016/0009-8981(70)90211-1.
Rustad P, Felding P, Franzson L, Kairisto V, Lahti A, Martensson A, Hyltoft Petersen P, Simonsson P, Steensland H, Uldall A. The Nordic Reference Interval Project 2000: recommended reference intervals for 25 common biochemical properties. Scand J Clin Lab Invest. 2004;64:271–84.
Cooper LB, Savarese G, Carrero JJ, et al. Clinical and research implications of serum versus plasma potassium measurements. Eur J Heart Fail. 2019;21(4):536–7. https://doi.org/10.1002/ejhf.1371.
Seamark D, Backhouse S, Barber P, Hichens J, Salzmann M, Powell R. Transport and temperature effects on measurement of serum and plasma potassium. J R Soc Med. 1999;92(7):339–41. https://doi.org/10.1177/014107689909200703).
Nijsten MW, de Smet BJ, Dofferhoff AS. Pseudohyperkalemia and platelet counts. N Engl J Med. 1991;325(15):1107. https://doi.org/10.1056/NEJM199110103251515).
Forsman RW. Why is the laboratory an afterthought for managed care organizations? Clin Chem. 1996;42:813–6.
Tromp J, van der Meer P. Hyperkalaemia: aetiology, epidemiology, and clinical significance. Eur Heart J Suppl. 2019;21(Suppl A):A6–11. https://doi.org/10.1093/eurheartj/suy028.
Humphrey T, Davids MR, Chothia MY, Pecoits-Filho R, Pollock C, James G. How common is hyperkalaemia? A systematic review and meta-analysis of the prevalence and incidence of hyperkalaemia reported in observational studies. Clin Kidney J. 2022;15(4):727–37. https://doi.org/10.1093/ckj/sfab243.
Martín-Pérez M, Ruigómez A, Michel A, García Rodríguez AL. Impact of hyperkalaemia definition on incidence assessment implications for epidemiological research based on a large cohort study in newly diagnosed heart failure patients in primary care. Bmc Fam Pract. 2016;17:51. https://doi.org/10.1186/s12875-016-0448-5.
Jain N, Kotla S, Little BB, Weideman RA, Brilakis ES, Reilly RF, Banerjee S. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol. 2012;15(109):1510–3. https://doi.org/10.1016/j.amjcard.2012.01.367.
Rossignol P, Lainscak M, Crespo-Leiro MG, et al. Unravelling the interplay between hyperkalaemia, renin-angiotensin aldosterone inhibitor use and clinical outcomes. Data from 9222 chronic heart failure patients of the ESC-HFA-EORPHeart Failure Long-Term Registry. Eur J Heart Fail. 2020;22:1378–89. https://doi.org/10.1002/ejhf.1793.
Fleet JL, Shariff SZ, Gandhi S, Weir MA, Jain AK, Garg AX. Validity of the International Classification of Diseases 10th revision code for hyperkalaemia in elderly patients at presentation to an emergency department and at hospital admission. BMJ Open. 2012;2(6):e002011. https://doi.org/10.1136/bmjopen-2012-002011.
Conway R, Creagh D, Byrne DG, O’Riordan D, Silke B. Serum potassium levels as an outcome determinant in acute medical admissions. Clin Med (Lond). 2015;15:239–43. https://doi.org/10.7861/clinmedicine.15-3-239.
Nilsson E, Gasparini A, Ärnlöv J, et al. Incidence and determinants of hyperkalemia and hypokalemia in a large healthcare system. Int J Cardiol. 2017;245:277–84. https://doi.org/10.1016/j.ijcard.2017.07.035.
Chang AR, Sang Y, Leddy J, et al. Antihypertensive medications and the prevalence of hyperkalemia in a large health system. Hypertension. 2016Jun;67(6):1181–8. https://doi.org/10.1161/HYPERTENSIONAHA.116.07363.
McMurray JJV, Ostergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767–71. https://doi.org/10.1016/S0140-6736(03)14283-3.
Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–67. https://doi.org/10.1056/NEJMoa0805450.
Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–92. https://doi.org/10.1056/NEJMoa1313731.
Khan SS, Campia U, Chioncel O, et al. EVEREST Trial Investigators. Changes in serum potassium levels during hospitalization in patients with worsening heart failure and reduced ejection fraction (from the EVEREST trial). Am J Cardiol. 2015;115(6):790–6. https://doi.org/10.1016/j.amjcard.2014.12.045.
Hunter RW, Bailey MA. Hyperkalaemia: pathophysiology, risk factors and consequences. Nephrol Dial Transplant. 2019;34(3):iii2–11. https://doi.org/10.1093/ndt/gfz206.
Palmer BF, Clegg DJ. Hyperkalaemia across the continuum of kidney function. Clin J Am Soc Nephrol. 2018;13:155–7. https://doi.org/10.2215/CJN.09340817.
Kovesdy CP, Matsushita K, Sang Y, et al. Serum potassium and adverse outcomes across the range of kidney function a ckd prognosis consortium metaanalysis. Eur Heart J. 2018;2018(39):1535–42. https://doi.org/10.1093/eurheartj/ehy100.
Raebel MA. Hyperkalaemia associated with use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Cardiovasc Ther. 2012;30(3):e156–66. https://doi.org/10.1111/j.1755-5922.2010.00258.x.
Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367(23):2204–13. https://doi.org/10.1056/NEJMoa1208799.
Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369(20):1892–903. https://doi.org/10.1056/NEJMoa1303154.
Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547–59. https://doi.org/10.1056/NEJMoa0801317.
Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;360:1953–2041. https://doi.org/10.1097/HJH.0000000000001940.
Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused up-date of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–61. https://doi.org/10.1161/CIR.0000000000000509.
Willenberg HS. How to escape from primary aldosteronism? News and views on an adrenal disorder of salt retention. Horm Metab Res. 2017Mar;49(3):151–63. https://doi.org/10.1055/s-0043-100767.
Markan U, Pasupuleti S, Pollard CM, Perez A, Aukszi B, Lymperopoulos A. The place of ARBs in heart failure therapy: is aldosterone suppression the key? Ther Adv Cardiovasc Dis. 2019;13:1–7. https://doi.org/10.1177/1753944719868134.
Azzam O, Nejad SH, Carnagarin R, Nolde JM, Galindo-Kiuchi M, Schlaich MP. Taming resistant hypertension: the promise of novel pharmacologic approaches and renal denervation. Br J Pharmacol. 2023Sep 15. https://doi.org/10.1111/bph.16247.
Wetmore JB, Yan H, Horne L, Peng Y, Gilbertson DT. Risk of hyperkalaemia from renin-angiotensin-aldosterone system inhibitors and factors associated with treatment discontinuities in a real-world population. Nephrol Dial Transplant. 2021;36(5):826–39. https://doi.org/10.1093/ndt/gfz263.
Weir MR, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol. 2010;5(3):531–48. https://doi.org/10.2215/CJN.07821109.
Pitt B, Bakris G, Ruilope LM, Di Carlo L, Mukherjee R. Serum potassium and clinical outcomes in the Eplerenon Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS). Circulation. 2008;118(16):543–51. https://doi.org/10.1161/CIRCULATIONAHA.108.778811.
Agarwal R, Joseph A, Anker SD, et al. Hyperkalaemia risk with finerenone: results from the FIDELIO-DKD trial. J Am Soc Nephrol. 2022;33(1):225–37. https://doi.org/10.1681/ASN.2021070942.
Avgerinos I, Karagiannis T, Matthews DR, Tsapas A, Bekiari E. Effects of sodium-glucose co-transporter-2 inhibitors by background cardiovascular medications: a systematic review and meta-analysis. Diabetes Obes Metab. 2023;25:3020–9. https://doi.org/10.1111/dom.15200.
Pochineni V, Rondon-Berrios H. Electrolyte and acid-base disorders in the renal transplant recipient. Front Med (Lausanne). 2018;5:261. https://doi.org/10.3389/fmed.2018.00261.
Lott C, Truhlář A, Alfonzo A, et al. European Resuscitation Council Guidelines 2021: cardiac arrest in special circumstances. Resuscitation. 2021;161:152–219. https://doi.org/10.1016/j.resuscitation.2021.02.011.
Luo J, Brunelli SM, Jensen DE, Yang A. Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol. 2016;11:90–100.
Aldahl M, Jensen AC, Davidsen L, Eriksen MA, Moller Hansen S, Nielsen BJ, Krogager ML, Kober L, Torp-Pedersen C, Sogaard P. Associations of serum potassium levels with mortality in chronic heart failure patients. Eur Heart J. 2017;38:2890–6.
Cooper LB, Benson L, Mentz RJ, Savarese G, DeVore AD, Carrero JJ, Dahlström U, Anker SD, Lainscak M, Hernandez A, Pitt B, Lund LH. Association between serum potassium level and outcomes in heart failure with reduced ejection fraction: a cohort study from the Swedish Heart Failure Registry. J Am Coll Cardiol. 2017;69(Suppl):678.
Beusekamp JC, Tromp J, van derWal HH, et al. Potassium and the use of renin–angiotensin–aldosterone system inhibitors in heart failure with reduced ejection fraction: data from BIOSTAT-CHF. Eur J Heart Fail. 2018;20:923–30. https://doi.org/10.1002/ejhf.1079.
Lund LH, Pitt B. Is hyperkalaemia in heart failure a risk factor or a risk marker? Implications for renin–angiotensin–aldosterone system inhibitor use. Eur J Heart Fail. 2018;20:931–2. https://doi.org/10.1002/ejhf.1175.
Rakisheva A, Marketou M, Klimenko A, Troyanova-Shchutskaia T, Vardas P. Hyperkalemia in heart failure: foe or friend? Clin Cardiol. 2020;43:666–75.
Tromp J, TerMaaten JM, Damman K, et al. Serum potassium levels and outcome in acute heart failure (data from the PROTECT and COACH trials). Am J Cardiol. 2017;119:290–6.
Bianchi S, Aucella F, De Nicola L, Genovesi S, Paoletti E, Regolisti G. Management of hyperkalaemia in patients with kidney disease: a position paper endorsed by the Italian Society of Nephrology. J Nephrol. 2019;32(4):499–516. https://doi.org/10.1007/s40620-019-00617-y.
Palmer BF. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what to do if the serum creatinine and/or serum potassium concentration rises. Nephrol Dial Transplant. 2003;18:1973–5. https://doi.org/10.1093/ndt/gfg282.
Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med. 2004;351:585–92. https://doi.org/10.1093/ndt/gfg282.
NCQA. Annual monitoring for patients on persistent medications: new measure for HEDIS. 2006. http://www.ncqa.org/Portals/0/HEDISQM/Archives/2006/Measureslist.pdf
Schepkens H, Vanholder R, Billiouw JM, Lameire N. Life-threatening hyperkalemia during combined therapy with angiotensin-converting enzyme inhibitors and spironolactone: an analysis of 25 cases. Am J Med. 2001;110:438–41. https://doi.org/10.1016/s0002-9343(01)00642-8.
Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]). Am J Cardiol. 1996;78(8):902–7. https://doi.org/10.1016/s0002-9149(96)00465-1
Harrison TN, Reynolds K, Hahn EE, et al. Laboratory monitoring to reduce adverse drug-related events: a mixed methods study. J Manag Care Spec Pharm. 2022;28(1):16–25. https://doi.org/10.18553/jmcp.2022.28.1.16.
Hollander-Rodriguez JC, Calvert JF Jr. Hyperkaliemia. Am Fam Physician. 2006;73(2):283–90.
Alvarez PJ, Brenner MS, Butler J, et al. Focus on Hyperkaliemia Management: expert consensus and economic impacts. J Manag Care Speciality Pharm. 2017;23(4):10-s20.
Lindner G, Burdmann EA, Clase CM, et al. Acute hyperkaliemia in the emergency department: a summary from kidney disease: Improving Global Outcomes conference. Eur J Emerg Med. 2020;27:329–37. https://doi.org/10.1097/MEJ.0000000000000691.
Davey M, Caldicott D. Calcium salts in management of hyperkalaemia. Emerg Med J. 2002Jan;19(1):92–3. https://doi.org/10.1136/emj.19.1.92-a.
Harel Z, Kamel KS. Optimal dose and method of administration of intravenous insulin in the management of emergency hyperkalemia: a systematic review. PLoS ONE. 2016;11(5):e0154963. https://doi.org/10.1371/journal.pone.0154963.
La Rue HA, Peksa GD, Shah SC. A comparison of insulin doses for the treatment of hyperkaliemia in patients with renal insufficiency. Pharmacotherapy. 2017;37:1516–22. https://doi.org/10.1002/phar.2038.
McNicholas BA, Pharm MH, Carli K, et al. Treatment of hyperkaliemia with a low-dose insulin protocol is effective and results in reduced hypoglycemia. Kidney Int Rep. 2018;3:328–36. https://doi.org/10.1016/j.ekir.2017.10.009.
Clase CM, Carrero JJ, Ellison DH, et al. Potassium homeostasis and management of dyskaliemia in kidney disease: conclusions from a Kidney Disease: Improving Global outcomes (KIDGO) Controversies Conference. Kidney Int. 2020;97:42–61. https://doi.org/10.1016/j.kint.2019.09.018.
Lehnhardt A, Kemper MJ. Pathogenesis, diagnosis and management of hyperkaliemia. Pediatric Nephrol. 2011;26(3):377–84. https://doi.org/10.1007/s00467-010-1699-3.
Scherr L, Ogden DA, Mead AW, Spritz N, Rubin AL. Management of hyperkalemia with a cation-exchange resin. N Engl J Med. 1961;264:115–9. https://doi.org/10.1056/NEJM196101192640303.
Parks M, Grady D. Sodium polystyrene sulfonate for hyperkalemia. JAMA Intern Med. 2019;179(8):1023–4. https://doi.org/10.1001/jamainternmed.2019.1291.
Laureati P, Xu Y, Trevisan M, et al. Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: a nationwide study. Nephrol Dial Transplant. 2020;35:1518–26. https://doi.org/10.1093/ndt/gfz150.
Li L, Harrison SD, Cope MJ, et al. Mechanism of action and pharmacology of patiromer, a nonabsorbed cross-linked polymer that lowers serum potassium concentration in patients with hyperkalemia. J Cardiovasc Pharmacol Ther. 2016;21:456–65. https://doi.org/10.1177/1074248416629549.
Bushinsky DA, Spiegel DM, Gross C, Benton WW, Fogli J, Hill Gallant KM, et al. Effect of patiromer on urinary ion excretion in healthy adults. Clin J Am Soc Nephrol. 2016;11:1769–76. https://doi.org/10.2215/CJN.01170216.
Buysse JM, Huang IZ, Pitt B. PEARL-HF: prevention of hyperkalemia in patients with heart failure using a novel polymeric potassium binder, RLY5016. Future Cardiol. 2012;8(1):17–28. https://doi.org/10.2217/fca.11.71.
Bakris GL, Pitt B, Weir MR, et al. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA. 2015;314(2):151–61. https://doi.org/10.1001/jama.2015.7446.
Weir MR, Bakris GL, Bushinsky DA, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372(3):211–21. https://doi.org/10.1056/NEJMoa1410853.
Butler J, Anker SD, Lund LH, et al. Patiromer for the management of hyperkalemia in heart failure with reduced ejection fraction: the DIAMOND trial. Eur Heart J. 2022;43(41):4362–73. https://doi.org/10.1093/eurheartj/ehac401.
Das S, Dey JK, Sen S, Mukherjee R. Efficacy and safety of patiromer in hyperkalemia: a systematic review and meta-analysis. J Pharm Pract. 2018Feb;31(1):6–17. https://doi.org/10.1177/0897190017692921.
Stavros F, Yang A, Leon A, Nuttall M, Rasmussen HS. Characterization of structure and function of ZS-9, a K+ selective ion trap. PLoS ONE. 2014;9(12): e114686. https://doi.org/10.1371/journal.pone.0114686.
Ash SR, Singh B, Lavin PT, Stavros F, Rasmussen HS. A phase 2 study on the treatment of hyperkalemia in patients with chronic kidney disease suggests that the selective potassium trap, ZS-9, is safe and efficient. Kidney Int. 2015;88(2):404–11. https://doi.org/10.1038/ki.2014.382.
Spinowitz BS, Fishbane S, Pergola PE, et al. Sodium zirconium cyclosilicate among individuals with hyperkalemia: a 12-month phase 3 study. Clin J Am Soc Nephrol. 2019;14(6):798–809. https://doi.org/10.2215/CJN.12651018.
Kosiborod M, Rasmussen HS, Lavin P, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312(21):2223–33. https://doi.org/10.1001/jama.2014.15688.
Roger SD, Spinowitz BS, Lerma EV, et al. Efficacy and safety of sodium zirconium cyclosilate for treatment of hyperkalemia: an 11-month open-label extension of HARMONIZE. Am J Nephrol. 2019;50(6):473–80. https://doi.org/10.1159/000504078.
Peacock WF, Rafique Z, Vishnevskiy K, et al. Emergency potassium normalization treatment including sodium zirconium cyclosilicate: a phase II, randomized, double-blind, placebo-controlled study (ENERGIZE). Acad Emerg Med. 2020;27(6):475–86. https://doi.org/10.1111/acem.13954.
Fishbane S, Ford M, Fukagawa M, et al. A phase 3b, randomized, double-blind, placebo-controlled study of sodium zirconium cyclosilicate for reducing the incidence of predialysis hyperkalemia. J Am Soc Nephrol. 2019;30(9):1723–33. https://doi.org/10.1681/ASN.2019050450.
Tardif JC, Rouleau J, Chertow GM, et al. Potassium reduction with sodium zirconium cyclosilicate in patients with heart failure. ESC Heart Fail. 2023;10(2):1066–76. https://doi.org/10.1002/ehf2.14268.
Murphy D, Ster IC, Kaski JC, Anderson L, Baneriee D. The LIFT Trial: study protocol for a double-blind randomized, placebo-controlled trial of K+ binder Lokelma for maximization of RAAS inhibition in CKD patients with heart failure. BMC Neprhol 2021; 22(1). https://doi.org/10.1186/s12882-021-02439-2.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Maurizio Giuseppe Abrignani, Edoardo Gronda, and Marco Marini. The first draft of the manuscript was written by Mauro Gori, Massimo Iacoviello, Pier Luigi Temporelli, Manuela Benvenuto, Giulio Binaghi, Arturo Cesaro, Alessandro Maloberti, and Maria Denitza Tinti, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abrignani, M.G., Gronda, E., Marini, M. et al. Hyperkalaemia in Cardiological Patients: New Solutions for an Old Problem. Cardiovasc Drugs Ther (2024). https://doi.org/10.1007/s10557-024-07551-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s10557-024-07551-7