Abstract
Background
Myocardial infarction remains a disease with high morbidity and death rate among cardiovascular diseases. Macrophages are abundant immune cells in the heart. Under different stimulatory factors, macrophages can differentiate into different phenotypes and play a dual pro-inflammatory and anti-inflammatory role. Therefore, a potential strategy for the treatment of myocardial infarction is to regulate the energy metabolism of macrophages and thereby regulate the polarization of macrophages. Tan IIA is an effective liposolubility component extracted from the root of Salvia miltiorrhiza and plays an important role in the treatment of cardiovascular diseases. On this basis, this study proposed whether Tan IIA could affect phenotype changes by regulating energy metabolism of macrophages, and thus exert its potential in the treatment of MI.
Methods
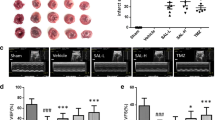
Establishing a myocardial infarction model, Tan IIA was given for 3 days and 7 days for intervention. Cardiac function was detected by echocardiography, and cardiac pathological sections of each group were stained with HE and Masson to observe the inflammatory cell infiltration and fibrosis area after administration. The expression and secretion of inflammatory factors in heart tissue and serum of each group, as well as the proportion of macrophages at the myocardial infarction site, were detected using RT-PCR, ELISA, and immunofluorescence. The mitochondrial function of macrophages was evaluated using JC-1, calcium ion concentration detection, reactive oxygen species detection, and mitochondrial electron microscopic analysis. Mechanically, single-cell transcriptome data mining, cell transcriptome sequencing, and molecular docking technology were used to anchor the target of Tan IIA and enrich the pathways to explore the mechanism of Tan IIA regulating macrophage energy metabolism and phenotype. The target of Tan IIA was further determined by gene knockdown and overexpression assay.
Results
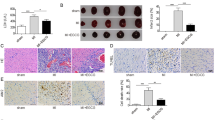
The intervention of Tan IIA can improve the cardiac function, inflammatory cell infiltration and fibrosis after MI, reduce the expression of inflammatory factors in the heart, enhance the secretion of anti-inflammatory factors, increase the proportion of M2-type macrophages, reduce the proportion of M1-type macrophages, and promote tissue repair, suggesting that Tan IIA has pharmacological effects in the treatment of MI. In terms of mechanism, RNA-seq results suggest that the phenotype of macrophages is strongly correlated with energy metabolism, and Tan IIA can regulate the PGK1-PDHK1 signaling pathway, change the energy metabolism mode of macrophages, and then affect its phenotype.
Conclusion
Tan IIA regulates the energy metabolism of macrophages and changes its phenotype through the PGK1-PDHK1 signaling pathway, thus playing a role in improving MI.






Similar content being viewed by others
Availability of Data and Material
All data are incorporated into the article and its online supplementary material. The sequencing data that support the findings of this study are openly available in Gene Expression Omnibus (GEO) at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE163465, reference number 163465.
Code Availability
Not applicable.
References
Ma Y, Mouton AJ, Lindsey ML. Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction. Trans Res. 2018;191:15–28. https://doi.org/10.1016/j.trsl.2017.10.001.
GBD 2019. Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors,1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol, 2021; 20: 795-820. https://doi.org/10.1016/S1474-4422(21)00252-0.
Mensah GA, Roth GA, Fuster V. The global burden of cardiovascular diseases and risk factors:2020 and beyond. J Am Coll Cardiol. 2019;74:2529–32. https://doi.org/10.1016/j.jacc.2019.10.009.
WHO. Cardiovascular diseases (CVDs) [EB/OL]. http://www.who.int/mediacentre/factsheets/fs317/en/. Accessed 2015-05-04.
Niccoli G, Scalone G, Lerman A, Crea F. Coronary microvascular obstruction in acute myocardial infarction. Eur Heart J. 2016;37:1024–33. https://doi.org/10.1093/eurheartj/ehv484.
Bowler MW. Conformational dynamics in phosphoglycerate kinase, an open and shut case? FEBS letters. 2013;587:1878–83. https://doi.org/10.1016/j.febslet.2013.05.012.
McCarrey JR, Thomas K. Human testis-specific PGK gene lacks introns and possesses characteristics of a processed gene. Nature. 1987;326:501–5. https://doi.org/10.1038/326501a0.
Wang S, Jiang B, Zhang T, et al. Insulin and mTOR pathway regulate HDAC3-mediated deacetylation and activation of PGK1. PLoS Biol. 2015;13:1–27. https://doi.org/10.1371/journal.pbio.1002243.
Hu H, Zhu W, Qin J, et al. Acetylation of PGK1 promotes liver cancer cell proliferation and tumorigenesis. Hepatology. 2017;65:515–28. https://doi.org/10.1002/hep.28887.
Nie H, Ju H, Fan J, et al. O-GlcNAcylation of PGK1 coordinates glycolysis and TCA cycle to promote tumor growth. Nat Commun. 2020;11:1–14. https://doi.org/10.1038/s41467-019-13601-8.
Tan Z, Xie N, Cui H, et al. Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism. J Immunol. 2015;194:6082–9. https://doi.org/10.4049/jimmunol.1402469.
Jin KY, Gao S, Yang PH, et al. Single-cell RNA sequencing reveals the temporal diversity and dynamics of cardiac immunity after myocardial infarction. Small Methods. 2022;6:1–15. https://doi.org/10.1002/smtd.202100752.
Ngoi N, Eu J, Hirpara J, et al. Targeting cell metabolism as cancer therapy. Antioxidants Redox Signal. 2020;32:285–308. https://doi.org/10.1089/ars.2019.7947.
Glatz JFC, Zuurbier CJ, Larsen TS. Targeting metabolic pathways to treat cardiovascular diseases. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165879. https://doi.org/10.1016/j.bbadis.2020.165879.
Yang Y, Shao M, Cheng W, et al. A pharmacological review of tanshinones, naturally occurring monomers from Salvia miltiorrhiza for the treatment of cardiovascular diseases. Oxid Med Cell Longev. 2023:3801908. https://doi.org/10.1155/2023/3801908.
Gao S, Liu Z, Li H, Little PJ, Liu P, Xu S. Cardiovascular actions and therapeutic potential of tanshinone IIA. Atherosclerosis. 2012;220:3–10. https://doi.org/10.1016/j.atherosclerosis.2011.06.041.
Guo R, Li L, Su J, et al. Pharmacological activity and mechanism of tanshinone IIA in related disease. Drug Des Devel Ther. 2020;14:4735–48. https://doi.org/10.2147/DDDT.S266911.
Fang Y, Duan C, Chen S, et al. Tanshinone-IIA inhibits myocardial infarct via decreasing of the mitochondrial apoptotic signaling pathway in myocardiocytes. Int J Mol Med. 2021;48:1–11. https://doi.org/10.3892/ijmm.2021.4991.
Li Q, Shen L, Wang Z, Jiang HP, Liu LX. Tanshinone IIA protects against myocardial ischemia reperfusion injury by activating the PI3K/Akt/mTOR signaling pathway. Biomed Pharmacother. 2016;84:106–14. https://doi.org/10.1016/j.biopha.2016.09.014.
Gao S, Wang Y, Li D, et al. Tanshinone IIA alleviates inflammatory response and directs macrophage polarization in lipopolysaccharide-stimulated RAW264.7 cells. Inflammation. 2019;42:264–75. https://doi.org/10.1007/s10753-018-0891-7.
Fan G, Jiang X, Wu X, et al. Anti-inflammatory activity of tanshinone IIA in LPS-stimulated RAW264.7 Macrophages via miRNAs and TLR4-NF-κB Pathway. Inflammation. 2016;39:375–84. https://doi.org/10.1007/s10753-015-0259-1.
Gao E, Lei Y, Shang X, et al. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res. 2010;107:1445–53. https://doi.org/10.1161/CIRCRESAHA.110.223925.
Chen J, Cao W, Aare P, et al. Amelioration of cardiac dysfunction and ventricular remodeling after myocardial infarction by danhong injection are critically contributed by anti-TGF-β-mediated fibrosis and angiogenesis mechanisms. J Ethnopharmacol. 2016;194:559–70. https://doi.org/10.1016/j.jep.2016.10.025.
Livnat-Levanon N, Glickman MH. Ubiquitin-proteasome system and mitochondria-reciprocity. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mech. 2011;1809:80–7. https://doi.org/10.1016/j.bbagrm.2010.07.005.
Arduino DM, Perocchi F. Pharmacological modulation of mitochondrial calcium homeostasis. J Physiol. 2018;596:2717–33. https://doi.org/10.1113/JP274959.
Ham PB, Raju R. Mitochondrial function in hypoxic ischemic injury and influence of aging. Prog Neurobiol. 2017;157:92–116. https://doi.org/10.1016/j.pneurobio.2016.06.006.
Bernardi P, Rasola A, Forte M, Lippe G. The mitochondrial permeability transition pore: channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. Physiol Rev. 2015;95:1111–55. https://doi.org/10.1152/physrev.00001.2015.
Jia D, Chen S, Bai P, et al. Cardiac resident macrophage-derived legumain improves cardiac repair by promoting clearance and degradation of apoptotic cardiomyocytes after myocardial infarction. Circulation. 2022;145:1542–56. https://doi.org/10.1161/CIRCULATIONAHA.121.057549.
Kieler M, Hofmann M, Schabbauer G. More than just protein building blocks: how amino acids and related metabolic pathways fuel macrophage polarization. FEBS J. 2021;288:3694–714. https://doi.org/10.1111/febs.15715.
Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1βthrough HIF-1α. Nature. 2013;496:238–42. https://doi.org/10.1038/nature11986.
Jan Y, Lai T, Yang C, Lin Y, Hsiao M. Adenylate kinase 4 modulates oxidative stress and stabilizes HIF-1α to drive lung adenocarcinoma metastasis. J Hematol Oncol. 2019;12:1–36. https://doi.org/10.1101/196857.
Wujak M, Veith C, Wu CY, et al. Adenylate kinase 4-a key regulator of proliferation and metabolic shift in human pulmonary arterial smooth muscle cells via Akt and HIF-1α signaling pathways. Int J Mol Sci. 2021;22:1–20. https://doi.org/10.3390/ijms221910371.
Chin WY, He CY, Chow TW, Yu QY, Lai LC, Miaw SC. Adenylate kinase 4 promotes inflammatory gene expression Hif1α and AMPK in macrophages. Front Immunol. 2021;12:630318. https://doi.org/10.3389/fimmu.2021.630318.
Chen B, Brickshawana A, Frangogiannis NG. The functional heterogeneity of resident cardiac macrophages in myocardial injury CCR2(+) cells promote inflammation, whereas CCR2(-) cells protect. Circulation Res. 2019;124(2):183–5. https://doi.org/10.1161/CIRCRESAHA.118.314357.
Bajpai G, Bredemeyer A, Li W, et al. Tissue resident CCR2- and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circul Res. 2019;124(2):263–78. https://doi.org/10.1161/CIRCRESAHA.118.314028.
Hilgendorf I, Gerhardt L, Tan T, et al. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circul Res. 2014;114(10):1611–22. https://doi.org/10.1161/CIRCRESAHA.114.303204.
Patel B, Ismahil MA, Hamid T, et al. Mononuclear phagocytes are dispensable for cardiac remodeling in established pressure-overload heart failure. PloS one. 2017;12(1): e0170781. https://doi.org/10.1371/journal.pone.0170781.
Dutta P, Sager HB, Stengel KR, et al. Myocardial infarction activates CCR2(+) hematopoietic stem and progenitor cells. Cell Stem Cell. 2015;16(5):477–87. https://doi.org/10.1016/j.stem.2015.04.008.
Nahrendorf M, Swirski FK, Aikawa E, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204(12):3037–47. https://doi.org/10.1084/jem.20070885.
Yap J, Irei J, Lozano-Gerona J, Vanapruks S, Bishop T, Boisvert WA. Macrophages in cardiac remodelling after myocardial infarction. Nat Rev Cardiol. 2023;20:373–85. https://doi.org/10.1038/s41569-022-00823-5.
Lin S, Zhang A, Yuan L, et al. Targeting parvalbumin promotes M2 macrophage polarization and energy expenditure in mice. Nat Commun. 2022;3301:1–15. https://doi.org/10.1038/s41467-022-30757-y.
Revelo XS, Parthiban P, Chen C, et al. Cardiac resident macrophages prevent fibrosis and stimulate angiogenesis. Circ Res. 2021;129(12):1086–101. https://doi.org/10.1161/CIRCRESAHA.121.319737.
Ardehali H, Sabbah HN, Burke MA, et al. Targeting myocardial substrate metabolism in heart failure: potential for new therapies. Eur J Heart Failure. 2012;14:120–9. https://doi.org/10.1093/eurjhf/hfr173.
Li X, Jiang Y, Meisenhelder J, et al. Mitochondria-translocated PGK1 functions as a protein kinase to coordinate glycolysis and the TCA cycle in tumorigenesis. Mol Cell. 2016;61:705–19. https://doi.org/10.1016/j.molcel.2016.02.009.
Qian X, Li X, Shi Z, et al. PTEN suppresses glycolysis by dephosphorylating and inhibiting autophosphorylated PGK1. Mol Cell. 2019;76:516–27. https://doi.org/10.1016/j.molcel.2019.08.006.
Funding
The authors gratefully acknowledge the support for this work from the National Natural Science Foundation of China (Grant No. 81904054 by S.G., 81774054 by C.L.) and Tianjin Municipal Health and Health Commission (Grant No. 2021103 by D.L.).
Author information
Authors and Affiliations
Contributions
S.G. and Z.Y. contributed equally to this work. S.G. designed and interpreted the experiments. Z.Y. and B.W. performed the cell experiments. Z.Y. and D.L. performed the animals’ experiments. X.Z. and C.L. performed all analysis. S.G. wrote the first draft. C.L. edited the manuscript and all authors provided comments on this manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
No competing interests declared.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors approved the final manuscript and the submission to this journal.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1122 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, S., Yang, Z., Li, D. et al. Intervention of Tanshinone IIA on the PGK1-PDHK1 Pathway to Reprogram Macrophage Phenotype After Myocardial Infarction. Cardiovasc Drugs Ther (2023). https://doi.org/10.1007/s10557-023-07520-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s10557-023-07520-6