Abstract
Purpose
The aim of this study was to evaluate the safety and efficacy of the Fantom BRS 6 months after implantation using the optical coherence tomography (OCT) imaging.
Methods
Twenty STEMI patients treated with a sirolimus‐eluting Fantom BRS were enrolled into a prospective, single-arm, serial observational study. The scaffold sizing, positioning and optimisation were guided by OCT imaging. The primary endpoint was device‐orientated composite endpoints (DOCE), comprised of cardiac death, target-vessel-related myocardial infarction and target lesion failure. To evaluate the device performance at the scaffold level, we performed a quantitative coronary angiography (QCA) and OCT imaging at 6 months.
Results
The primary endpoint did not occur in any patient within the 6-month follow‐up. There were no major adverse cardiac events (MACEs) or DOCEs, no cases of scaffold thrombosis, target lesion revascularization and no deaths. In QCA, we observed a decrease in the minimum and mean lumen diameter in the in-scaffold region and in the proximal and distal peri-scaffold region. Similarly, the minimum lumen area and reference vessel diameter had decreased in both QCA and OCT. The OCT imaging showed improvement in the expansion index and malposition rate.
Conclusion
A serial 6-month OCT imaging after implantation of a third-generation Tyrocore-based bioresorbable coronary scaffold indicated good coverage of the struts with excellent healing of the scaffold, low neointima growth and no signs of neoatherosclerosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Upon its introduction, the bioresorbable vascular scaffold (BVS) (Absorb™; Abbott Vascular, Santa Clara, CA, USA) was declared a transformative technology designed to restore coronary structure and functionality and to enhance the long-term outcomes of metallic stents by overcoming the dangers of stent thrombosis, stent fracture and neoatherosclerosis. These hopes were not confirmed by the early and very late results of randomised controlled trials that compared the BVS with the XIENCE everolimus-eluting stent (Abbott Vascular), which demonstrated increased rates of scaffold thrombosis and target lesion failure (TLF) after 1, 2, 4 and 5 years [1,2,3,4]. These safety concerns led to a cessation of the commercialisation of the product and to the European Society of Cardiology guidelines downgrading the recommendation for the use of BVSs to class III [5]. Still, insights from other clinical programmes evaluating next-generation coronary scaffolds suggest that improvement of the technology, especially in regard to the mechanical and biocompatibility properties of the polymer, is needed to justify its clinical use [6,7,8]. The Fantom (REVA Medical, USA) is a third-generation bioresorbable scaffold (BRS) made with Tyrocore, a new polymer composed mainly of an iodinated short-chain polycarbonate copolymer of tyrosine analogues and characterised by less release of lactic acid, less irritation, much-diminished tissue calcium formation and improved endothelisation in comparison to Absorb [9]. Long-term follow-up of Fantom BRS in chronic coronary syndrome showed sustained safety and efficacy (TLF = 5.4%; major adverse cardiac events [MACE] = 5.8%) after 48 months) that is comparable to contemporary drug-eluting stents (DES) [10].
ST-segment elevation myocardial infarction (STEMI) has been identified as one of the more favourable scenarios for the use of BVS technology for primary percutaneous coronary intervention (pPCI), since (1) the patients are usually younger at admission, with a high likelihood of repeat intervention over the lifespan, and thus may have greater benefit from cage-free coronary vessels, (2) their plaques are ruptured, soft and thrombotic, and (3) the strong mechanical support of the vessel wall is less of importance as the fibro-calcific morphology is rare [11].
To date, no clinical studies have assessed arterial healing of third-generation Fantom Encore BRS implantation in the thrombogenic milieu of a STEMI setting. Therefore, the purpose of the Fantom STEMI pilot project was to obtain a serial prospective follow-up and to evaluate the healing process over 36 months after pPCI. Herein, we present the results of the early phase after a 6-month clinical, angiographic and intravascular imaging follow-up was conducted.
Methods
Device, Patients, and Study Design
This is a single-centre, investigator-initiated prospective registry of STEMI patients (clinicaltrial.org, NCT03785431) treated with the Fantom device. The Fantom is the third-generation balloon-expandable, CE-marked BRS. It is characterised by a thin strut profile (95–115 μm) and is made of Tyrocore with enhanced radiopacity. The details of the device have been described previously [12].
At baseline, 20 patients underwent optical coherence tomography (OCT)-guided pPCI with Fantom Encore implantation. The inclusion and exclusion criteria as well as the procedural protocol have been reported elsewhere [12]. In brief, adults with typical chest pain onset of less than 12 h and electrocardiographic confirmation of ST-segment elevation were recruited. The angiographic inclusion criteria included a de novo lesion in a native coronary artery and visually estimated stenosis of at least 50% with a reference vessel diameter (RVD) of between 2.5 and 3.5 mm and a lesion length of up to 20 mm. The exclusion criteria were cardiogenic shock, pulmonary oedema, known hypersensitivity to or contraindications for antiplatelet/antithrombotic medications or contrast agents and specific lesion locations or characteristics (non-native vessel, left main coronary artery, true bifurcation and significant calcifications). A 12-month dual antiplatelet therapy with aspirin and ticagrelor was recommended.
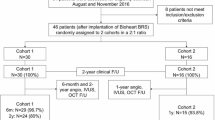
At 6 months, the patients were scheduled for hospital admission for clinical and invasive assessment with coronary angiography and intravascular imaging using OCT. The study flow chart is presented in Fig. 1. The local ethics committee approved the protocol following the Declaration of Helsinki.
Study Endpoints and Definitions
The study endpoints have been described previously [12]. In brief, the primary device-orientated composite endpoints (DOCE) consisted of cardiac death, target-vessel MI or ischemia-driven TLR at 180 days. MACEs included nonfatal MI, TLR, target-vessel revascularization (TVR) and cardiovascular death. Patient-orientated composite endpoints (POCE) consisted of all-cause mortality, any myocardial infarction and repeat revascularisation [13]. Additionally, an angiographic and OCT analysis were performed.
Baseline Procedure and Follow-Up Intravascular Imaging
The pPCI was performed according to the previously published study protocol [12]. In brief, the patients provided informed consent in the CathLab before the procedure. Both radial and femoral access were allowed. The angiographic eligibility criteria were checked in the 2 near orthogonal projections of the target segment. At the operator’s discretion, manual aspiration thrombectomy or an undersized balloon to restore the blood flow (at least TIMI 2) were allowed. An OCT pullback was performed for sizing and lesion morphology assessment, followed by predilatation with a 1:1 noncompliant or semicompliant balloon. Post-dilatation was mandatory to achieve optimal scaffold expansion in OCT. At 6 months, an in-hospital invasive follow-up with intravascular OCT and angiography analysis was carried out in all patients.
Angiographic and OCT Imaging Analysis
An off-line qualitative and quantitative coronary angiography (QCA) and OCT were analysed by an independent core laboratory (Krakow Cardiovascular Research Institute, Krakow, Poland) using previously reported methodology [12]. In brief, the following QCA parameters were assessed in the scaffolded segment: RVD, minimal lumen diameter (MLD), diameter stenosis (%DS), minimum lumen area and late lumen loss (LLL).
The OCT imaging was performed with a Dragonfly™ OPTIS™ catheter (Abbott Vascular, Santa Clara, CA, USA) according to standard procedures and was analysed at an independent core laboratory (Krakow Cardiovascular Research Institute, Krakow) by analysts blinded to the angiographic data and clinical characteristics using proprietary LightLab off-line analytical software. The analysis was performed in the in-scaffold segments (IS) and out-scaffold segments (OS) (5 mm adjacent to the proximal and distal segments from the radiopaque platinum markers) [14]. The qualitative and quantitative OCT analyses are described in the Supplementary Appendix.
Statistical Analysis
The primary endpoint and all imaging findings (OCT and angiography) were analysed based on the as-treated population. The data distribution was assessed by Kolmogorov–Smirnov analysis. The continuous variables are presented as mean ± standard deviation (SD) for those with normal distributions or as median and interquartile range (IQR) for those with non-normal distribution. The categorical variables are presented as counts and percentages. The Wilcoxon signed-rank test was used for 2 related sample comparisons; the Pearson correlation coefficient was used for continuous variables. Mixed models were used to consider the clustered nature of OCT data regarding struts, malposition, segments and edges. Statistical analysis was performed using JMP software, version 9.0.0 (SAS Institute, Cary, NC, USA).
Results
Overall, 20 patients were enrolled in the study, and 19 patients underwent clinical and invasive follow-up at 6 months. One patient, who did not report any MACEs and felt well, withdrew their informed consent for invasive imaging (Fig. 1). The demographic and clinical characteristics at baseline are shown in Table 1. The mean age was 61.26 ± 9.48 years; there were 9 women (47.4%).
The details of the procedures are summarized in Table 2. The procedures were performed via radial (n = 17; 89.5%) and femoral (n = 2; 10.5%) access. A single scaffold was used in each patient. Manual aspiration thrombectomy was performed in 2 cases (10.5%). The mean stent diameter was 2.42 ± 0.56 mm, and the mean stent length was 14.42 ± 2.43 mm. The actual mean deployment pressure was 10.32 ± 2.78 atm. All patients received a dual antiplatelet therapy consisting of aspirin (100%); 13 patients received ticagrelor (68.4%), while the remaining 6 patients received a P2Y12 inhibitor, that is, clopidogrel (31.6%). An unfractionated heparin (UFH) was used in all cases.
Overall, at the 6-month follow-up, there were no events of the primary endpoint, defined as DOCE (cardiac death, target-vessel MI or TLR). No MACEs (MI, TLR, TVR or cardiac death) or cases of scaffold thrombosis were reported either. A POCE (death, MI or repeat PCI) was reported in 1 patient who underwent elective staged PCI of the left anterior descending artery with DES implantation. The details are presented in Table 3.
Quantitative Coronary Angiography Analysis
The results from QCA analysis at baseline and at 180 days are shown in Table 4. We observed a statistically significant increase in %DS (5.28% ± 4.40% vs 9.39% ± 5.78%, p < 0.05) with a decrease in MLD (2.88 ± 0.35 mm vs 2.68 ± 0.38 mm, p < 0.01), minimum lumen area (6.58 ± 1.53 mm2 vs 5.74 ± 1.52 mm2, p < 0.01) and RVD (3.04 ± 0.36 mm vs 2.95 ± 0.36 mm, p < 0.05). The LLL at 6 months was 0.20 ± 0.25 mm.
Quantitative and Qualitative OCT Analysis
The results from quantitative and qualitative OCT analysis at baseline and 180 days are shown in Table 5. Eighteen patients underwent OCT imaging of the index vessel at 6 months. The paired quantitative OCT analysis revealed that the minimum lumen area and the mean lumen area had decreased over the 6 months by 1.55 mm2 (6.61 ± 1.92 mm2 vs 5.06 ± 1.60 mm2, p < 0.0001) and by 1.61 mm2 (7.97 ± 2.13 mm2 vs 6.36 ± 1.78 mm2, p < 0.0001), respectively. We found no change in the mean scaffold area (8.16 ± 2.18 mm2 vs 8.07 ± 1.97 mm2, p = 0.44) (Fig. 2).
The distal reference mean lumen area decreased by 0.76 mm2 (6.78 ± 2.77 mm2 vs 6.02 ± 2.30 mm2, p < 0.05). There was no statistically significant change in proximal reference mean lumen area (8.62 ± 3.97 mm2 vs 7.62 ± 2.75 mm2, p = 0.08). The number of scaffolds with incomplete stent apposition decreased from 10 (58.2%) to 2 (11.76%) (p < 0.05). The rate of malapposed struts decreased from 0.46% at baseline to 0% (p < 0.05) 6 months after the procedure. The mean neointima thickness at 6 months was 177 µm (161–215 µm) with 0.23 (0.19–0.29) symmetry. The lumen asymmetry index (AI) decreased by 0.03 (0.33 ± 0.07 vs 0.36 ± 0.07, p < 0.05). We observed no significant change in the lumen eccentricity index (EI) (0.86 ± 0.02 vs 0.85 ± 0.03, p = 0.09) (Fig. 3).
None of the scaffolds had any atherosclerotic findings at the 6-month follow-up. The mean peri-strut low-intensity area (PLIA) score 6 months after the procedure was 2.00 (0.60–2.71). An example of the vascular response in OCT is presented in Fig. 4.
Discussion
The Fantom is a novel third-generation BRS characterised by thin struts, good expansion capability and radiopacity. Most of the studies on the Fantom BRS included patients with stable coronary artery disease. The data on the use of BRS in patients with STEMI are limited, despite the fact that this group of patients may obtain many benefits from BRS implantation. Coronary artery lesions in STEMI patients are often localised in the proximal part of the coronary artery, so restoration of vasomotion may have a greater effect than in patients with stable disease. Due to the necrotic core of the plaque in STEMI lesions, metallic stent implantation interferes with vascular healing and leads to coronary evagination and malapposition. BRS implantation may prevent these complications [15, 16].
The major findings of the current study are as follows:
-
1.
Fantom BRS implantation in patients with STEMI was safe, with no MACEs or DOCEs 6 months after the procedure.
-
2.
We observed an expected steady decrease in minimum lumen area and mean lumen area.
-
3.
OCT showed a decrease in the rate of strut malapposition and an improvement in lumen asymmetry.
Increased platelet activation and vasoconstriction are major concerns resulting from the implantation of a BRS. The results of the Fantom pilot study on 10 patients with STEMI showed that no patient had any DOCEs during a 30-day follow-up period [12]. In the current study, entirely guided by OCT, we observed that Fantom BRS was safe. The primary DOCE—consisting of cardiac death, target vessel-related MI and TLR—did not occur during the 6 months of the study. The TROFI II study compared the safety and efficacy of the first-generation Absorb stents and the metallic everolimus-eluting stent in patients with STEMI. Within 6 months of implantation, DOCE occurred in 1 patient (1.1%) in the Absorb arm; it was a subacute definite stent thrombosis leading to MI and clinically-driven TLR [17]. Also, there were no MACEs in our study and no cases of stent thrombosis. In the Fantom II study with 108 patients, 3 MACEs (2.8%) were recorded with 1 case (0.9%) of stent thrombosis [18].
Despite MLA and mean lumen area decreasing after 6 months, the Fantom BRS maintained a stable minimum and mean scaffold area. Similar results were obtained in the FANTOM II study [19].
According to Asano et al., LLL may be a predictor of TLR in patients after various angioplasty techniques. The Kaplan–Meier analysis showed that the incidence rate of TLR in the patients with an LLL over 0.50 mm was 24.6% at the 4-year follow-up angiography, while in the patients with an LLL of 0.50 mm or greater was 4.6% [20]. In our study, the LLL was 0.20 mm ± 0.25 mm, while in the Fantom II study, it was 0.25 ± 0.40 mm [18]. The LLL values at 6 months with metallic DESs were 0.11 ± 0.23 mm with everolimus-eluting stents [21], 0.36 ± 0.48 mm with paclitaxel-eluting stents [22] and 0.61 ± 0.46 mm with zotarolimus-eluting stents (after 8 months) [23].
In the present study, the frequency of PLIA was 76.47% with a mean value of 2.00 (0.60–2.71). This may be related to arterial healing and local vessel injury. Sato et al. suggested that the appearance of PLIA on OCT up to 1 year after BRS implantation may be related to the biological processes occurring within the scaffold, as with DESs. This may resolve after 3 or 4 years, when the resorption process has been completed, as opposed to metallic stents [24].
There are obvious limitations due to the single-arm, non-randomised study design and the limited number of patients in one centre. However, to date, this is the only longitudinal study with serial, long-term OCT follow-up of the Fantom BRS in STEMI patients.
In conclusion, serial 6-month OCT imaging after implantation of the third-generation Tyrocore-based bioresorbable coronary scaffold showed no significant safety concerns, with good coverage of the struts along with excellent healing of the scaffold and low neointima growth and no signs of neoatherosclerosis. Still, given a small study sample, these results warrant to be confirmed in a larger trial, prior drawing firm conclusions.
Impact on Daily Practice
Implantation of Fantom BRS is related to a low risk of occurrence of MACE or DOCE and scaffold thrombosis. The resorption of the scaffold with a low neointima growth may improve long-term outcomes and future treatment for patients.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ali ZA, Serruys PW, Kimura T, Gao R, Ellis SG, Kereiakes DJ, Onuma Y, Simonton C, Zhang Z, Stone GW. 2-year outcomes with the absorb bioresorbable scaffold for treatment of coronary artery disease: a systematic review and meta-analysis of seven randomised trials with an individual patient data substudy. Lancet. 2017;390(10096):760–72.
Chevalier B, Cequier A, Dudek D, Haude M, Carrie D, Sabate M, Windecker S, Reith S, de Sousa Almeida M, Campo G, Iniguez A, Onuma Y, Serruys PW. Four-year follow-up of the randomised comparison between an everolimus-eluting bioresorbable scaffold and an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II Trial). EuroIntervention. 2018;13(13):1561–4.
Onuma Y, Chevalier B, Ono M, Cequier A, Dudek D, Haude M, Carrie D, Sabate M, Windecker S, Rapoza RR, West NEJ, Reith S, de Sousa Almeida M, Campo G, Iniguez-Romo A, Serruys PW. Bioresorbable scaffolds versus everolimus-eluting metallic stents: five-year clinical outcomes of the randomised ABSORB II trial. EuroIntervention. 2020;16(11):e938–41.
Serruys PW, Chevalier B, Dudek D, Cequier A, Carrie D, Iniguez A, Dominici M, van der Schaaf RJ, Haude M, Wasungu L, Veldhof S, Peng L, Staehr P, Grundeken MJ, Ishibashi Y, Garcia-Garcia HM, Onuma Y. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet. 2015;385(9962):43–54.
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ, E.S.C.S.D. Group. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–77.
Gomez-Lara J, Ortega-Paz L, Brugaletta S, Cuesta J, Romani S, Serra A, Salinas P, Garcia Del Blanco B, Goicolea J, Hernandez-Antolin R, Antuna P, Romaguera R, Regueiro A, Rivero F, Cequier A, Alfonso F, Gomez-Hospital JA, Sabate M. Collaborators, Bioresorbable scaffolds versus permanent sirolimus-eluting stents in patients with ST-segment elevation myocardial infarction: vascular healing outcomes from the MAGSTEMI trial. EuroIntervention. 2020;16(11):e913–21.
Haude M, Ince H, Abizaid A, Toelg R, Lemos PA, von Birgelen C, Christiansen EH, Wijns W, Neumann FJ, Kaiser C, Eeckhout E, Lim ST, Escaned J, Onuma Y, Garcia-Garcia HM, Waksman R. Sustained safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de novo coronary lesions: 12-month clinical results and angiographic findings of the BIOSOLVE-II first-in-man trial. Eur Heart J. 2016;37(35):2701–9.
Kochman J, Koltowski L, Tomaniak M, Jakala J, Proniewska K, Legutko J, Roleder T, Piertrasik A, Rdzanek A, Kochman W, Brugaletta S, Opolski G, Regar E. First serial optical coherence tomography assessment at baseline, 12 and 24 months in STEMI patients treated with the second-generation absorb bioresorbable vascular scaffold. EuroIntervention. 2018;13(18):e2201–9.
Leibundgut G. A novel, radiopaque, bioresorbable tyrosine-derived polymer for cardiovascular scaffolds, cardiac interventions today, vol., no 2018, p. European Featured Technology. 2018. Available online: https://citoday.com/articles/2018-july-aug/a-novel-radiopaque-bioresorbable-tyrosine-derived-polymer-for-cardiovascular-scaffolds?c4src=archive:feed. Accessed 29 Feb 2020.
Lutz M, Anderson J, Abizaid A, et al. TCT CONNECT-270 FANTOM II Trial: Safety and performance study of the fantom sirolimus-eluting bioresorbable coronary scaffold—first report: 4-year clinical outcomes. J Am Coll Cardiol. 2020;76(17 Supplement S):B118.
Kajiya T, Liang M, Sharma RK, Lee CH, Chan MY, Tay E, Chan KH, Tan HC, Low AF. Everolimus-eluting bioresorbable vascular scaffold (BVS) implantation in patients with ST-segment elevation myocardial infarction (STEMI). EuroIntervention. 2013;9(4):501–4.
Koltowski L, Tomaniak M, Ochijewicz D, Maksym J, Roleder T, Zaleska M, Proniewska K, Opolski G, Kochman J. Second generation, sirolimus-eluting, bioresorbable Tyrocore scaffold implantation in patients with ST-segment elevation myocardial infarction: baseline OCT and 30-day clinical outcomes - A FANTOM STEMI pilot study. Catheter Cardiovasc Interv. 2020;96(1):E1–7.
Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW, C Academic research. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344–51.
Serruys PW, Ormiston JA, Onuma Y, Regar E, Gonzalo N, Garcia-Garcia HM, Nieman K, Bruining N, Dorange C, Miquel-Hebert K, Veldhof S, Webster M, Thuesen L, Dudek D. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet. 2009;373(9667):897–910.
Hong MK, Mintz GS, Lee CW, Kim YH, Lee SW, Song JM, Han KH, Kang DH, Song JK, Kim JJ, Park SW, Park SJ. Incidence, mechanism, predictors, and long-term prognosis of late stent malapposition after bare-metal stent implantation. Circulation. 2004;109(7):881–6.
Scalone G, Brugaletta S, Gomez-Monterrosas O, Otsuki S, Sabate M. ST-segment elevation myocardial infarction - ideal scenario for bioresorbable vascular scaffold implantation? Circ J. 2015;79(2):263–70.
Sabate M, Windecker S, Iniguez A, Okkels-Jensen L, Cequier A, Brugaletta S, Hofma SH, Raber L, Christiansen EH, Suttorp M, Pilgrim T, Anne van Es G, Sotomi Y, Garcia-Garcia HM, Onuma Y, Serruys PW. Everolimus-eluting bioresorbable stent vs. durable polymer everolimus-eluting metallic stent in patients with ST-segment elevation myocardial infarction: results of the randomized ABSORB ST-segment elevation myocardial infarction-TROFI II trial. Eur Heart J. 2016;37(3):229–40.
Abizaid A, Carrie D, Frey N, Lutz M, Weber-Albers J, Dudek D, Chevalier B, Weng SC, Costa RA, Anderson J, Stone GW, Investigators FIC. 6-month clinical and angiographic outcomes of a novel radiopaque sirolimus-eluting bioresorbable vascular scaffold: the FANTOM II study. JACC Cardiovasc Interv. 2017;10(18):1832–8.
Simonsen JK, Holck EN, Carrie D, Frey N, Lutz M, Weber-Albers J, Dudek D, Chevalier B, Daemen J, Dijkstra J, Fox Maule C, Neghabat O, Lassen JF, Anderson J, Christiansen EH, Abizaid A, Holm NR. Mechanical performance and healing patterns of the novel sirolimus-eluting bioresorbable Fantom scaffold: 6-month and 9-month follow-up by optical coherence tomography in the FANTOM II study. Open Heart. 2019;6(1):e000941.
Asano T, Serruys PW, Collet C, Miyazaki Y, Takahashi K, Chichareon P, Katagiri Y, Modolo R, Tenekecioglu E, Morel MA, Garg S, Wykrzykowska J, Piek JJ, Sabate M, Morice MC, Chevalier B, Windecker S, Onuma Y. Angiographic late lumen loss revisited: impact on long-term target lesion revascularization. Eur Heart J. 2018;39(36):3381–9.
Grube E, Sonoda S, Ikeno F, Honda Y, Kar S, Chan C, Gerckens U, Lansky AJ, Fitzgerald PJ. Six- and twelve-month results from first human experience using everolimus-eluting stents with bioabsorbable polymer. Circulation. 2004;109(18):2168–71.
Grube E, Silber S, Hauptmann KE, Mueller R, Buellesfeld L, Gerckens U, Russell ME. TAXUS I: six- and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions. Circulation. 2003;107(1):38–42.
Fajadet J, Wijns W, Laarman GJ, Kuck KH, Ormiston J, Munzel T, Popma JJ, Fitzgerald PJ, Bonan R, Kuntz RE, Investigators EI. Randomized, double-blind, multicenter study of the endeavor zotarolimus-eluting phosphorylcholine-encapsulated stent for treatment of native coronary artery lesions: clinical and angiographic results of the ENDEAVOR II trial. Circulation. 2006;114(8):798–806.
Sato T, Jose J, El-Mawardy M, Sulimov DS, Tölg R, Richardt G, Abdel-Wahab M. Relationship between peri-strut low intensity areas and vascular healing response after everolimus-eluting bioresorbable scaffold implantation: an optical coherence tomography study. J Cardiol. 2017;69(4):606–12.
Lucas CM, Harris RJ, Giannoudis A, Davies A, Knight K, Watmough SJ, Wang L, Clark RE. Chronic myeloid leukemia patients with the e13a2 BCR-ABL fusion transcript have inferior responses to imatinib compared to patients with the e14a2 transcript. Haematologica. 2009;94(10):1362–7.
Acknowledgements
Editorial assistance was provided by Proper Medical Writing, Warsaw, Poland.
Author information
Authors and Affiliations
Contributions
LK and JK performed invasive procedures including scaffold implantations and follow-up imaging. LK, MT and DO collected the clinical data. LK, MT. JK and GO designed the study protocol. All authors analyzed and interpreted the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The local ethics committee approved the study protocol. This study was performed in line with the principles of the Declaration of Helsinki.
Conflict of Interest
Łukasz Kołtowski received research grants and speaker’s fees from Reva Medical and speaker’s fees from Abbott. All other authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koltowski, L., Tomaniak, M., Ochijewicz, D. et al. Third-Generation Sirolimus‐Eluting Bioresorbable Tyrocore Scaffold Implantation in Patients with ST‐Segment Elevation Myocardial Infarction: Baseline and 6-Month OCT and Clinical Outcomes—a FANTOM STEMI Pilot Study. Cardiovasc Drugs Ther (2023). https://doi.org/10.1007/s10557-023-07429-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s10557-023-07429-0