Abstract
Purpose
The benefits of statins for ischemic cardio-cerebrovascular diseases are well known. However, concerns around muscle adverse events still exist. We therefore aimed to compare the muscle safety of individual statins in adults.
Methods
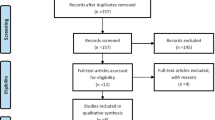
PubMed, Embase, Cochrane Central Register of Controlled Trials and Web of Science were searched to include double-blind randomized controlled trials (RCTs) comparing one statin with another or with control treatment. Pairwise meta-analyses and network meta-analyses were undertaken with Stata 14.0 software. Relative risk (RR) with 95% confidence intervals (CIs) was adopted for each outcome.
Results
A total of 83 RCTs were included. In the pairwise meta-analysis, statins were significantly associated with only a slight increase in muscle symptoms compared with control (RR=1.05; 95% CI=1.01–1.09). In the drug-level network meta-analyses, no statistically significant difference was found between individual statins in the incidence of muscle symptoms, myalgia, myopathy, rhabdomyolysis, creatine kinase (CK) >10 times the upper limit of normal (ULN) or discontinuation due to muscle adverse events. In the dose-level network meta-analyses, there were no statistically significant dose-dependent effects on any outcomes except that moderate-intensity statins had a higher incidence of muscle symptoms than control (RR=1.13; 95% CI=1.01–1.27). Moderate simvastatin (RR=6.57; 95% CI=1.26–34.41) and moderate pravastatin (RR=5.96; 95% CI=1.00–35.44) had a statistically significantly higher incidence of CK >10×ULN compared with moderate atorvastatin. Lipophilic statins and statins metabolized by liver cytochrome P450 3A4 were not associated with an increased risk of muscle adverse events.
Conclusion
Statins may be generally safe on muscle. Moderate atorvastatin may be superior to equivalent simvastatin and pravastatin in muscle tolerability.




Similar content being viewed by others
Data Availability
All the data supporting our findings are presented in this published article and its Supplementary Information files.
Abbreviations
- CI :
-
Confidence interval
- CK :
-
Creatine kinase
- ULN :
-
Upper limit of normal
- CYP3A4 :
-
Cytochrome P450 3A4
- CENTRAL :
-
Cochrane Central Register of Controlled Trials
- IF :
-
Inconsistency factor
- MeSH :
-
Medical Subject Headings
- NFP :
-
Network forest plot
- RCT :
-
Randomized controlled trial
- RR :
-
Relative risk
- SUCRA :
-
Surface under the cumulative ranking curve
References
Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol Med. 2008;14(1):37–44.
Al-Mohaissen MA, Ignaszewski MJ, Frohlich J, Ignaszewski AP. Statin-Associated Muscle Adverse Events: Update for clinicians. Sultan Qaboos Univ Med J. 2016;16(4):e406–e15.
Auer J, Sinzinger H, Franklin B, Berent R. Muscle- and skeletal-related side-effects of statins: tip of the iceberg? Eur J Prev Cardiol. 2016;23(1):88–110.
Li JJ, Liu HH, Wu NQ, Yeo KK, Tan K, Ako J, et al. Statin intolerance: an updated, narrative review mainly focusing on muscle adverse effects. Expert Opin Drug Metab Toxicol. 2020;16(9):837–51.
Nissen SE, Stroes E, Dent-Acosta RE, Rosenson RS, Lehman SJ, Sattar N, et al. Efficacy and Tolerability of Evolocumab vs Ezetimibe in Patients With Muscle-Related Statin Intolerance: The GAUSS-3 Randomized Clinical Trial. Jama. 2016;315(15):1580–90.
Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012–22.
Rosenson RS, Baker SK, Jacobson TA, Kopecky SL, Parker BA. The National Lipid Association's Muscle Safety Expert P. An assessment by the Statin Muscle Safety Task Force: 2014 update. J Clin Lipidol. 2014;8(3 Suppl):S58–71.
Wu Y, Lach B, Provias JP, Tarnopolsky MA, Baker SK. Statin-associated Autoimmune Myopathies: A Pathophysiologic Spectrum. Can J Neurol Sci. 2014;41(5):638–47.
Alfirevic A, Neely D, Armitage J, Chinoy H, Cooper RG, Laaksonen R, et al. Phenotype standardization for statin-induced myotoxicity. Clin Pharmacol Ther. 2014;96(4):470–6.
Irwin JC, Khalesi S, Fenning AS, Vella RK. The effect of lipophilicity and dose on the frequency of statin-associated muscle symptoms: A systematic review and meta-analysis. Pharmacol Res. 2018;128:264–73.
Climent E, Benaiges D, Pedro-Botet J. Hydrophilic or Lipophilic Statins? Front Cardiovasc Med. 2021;8:687585.
Hirota T, Ieiri I. Drug-drug interactions that interfere with statin metabolism. Expert Opin Drug Metab Toxicol. 2015;11(9):1435–47.
Silva MA, Swanson AC, Gandhi PJ, Tataronis GR. Statin-related adverse events: a meta-analysis. Clin Ther. 2006;28(1):26–35.
Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Intern Emerg Med. 2017;12(1):103–11.
Guyton JR, Bays HE, Grundy SM, Jacobson TA. The National Lipid Association Statin Intolerance P. An assessment by the Statin Intolerance Panel: 2014 update. J Clin Lipidol. 2014;8(3 Suppl):S72–81.
Banach M, Serban C, Sahebkar A, Ursoniu S, Rysz J, Muntner P, et al. Effects of coenzyme Q10 on statin-induced myopathy: a meta-analysis of randomized controlled trials. Mayo Clin Proc. 2015;90(1):24–34.
Newman CB, Preiss D, Tobert JA, Jacobson TA, Page RL 2nd, Goldstein LB, et al. Statin Safety and Associated Adverse Events: A Scientific Statement From the American Heart Association. Arterioscler Thromb Vasc Biol. 2019;39(2):e38–81.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed). 2011;343:d5928.
Shim S, Yoon BH, Shin IS, Bae JM. Network meta-analysis: application and practice using Stata. Epidemiol Health. 2017;39:e2017047.
Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G. Evaluation of inconsistency in networks of interventions. Int J Epidemiol. 2013;42(1):332–45.
Chaimani A, Salanti G, Leucht S, Geddes JR, Cipriani A. Common pitfalls and mistakes in the set-up, analysis and interpretation of results in network meta-analysis: what clinicians should look for in a published article. Evid-Based Mental Health. 2017;20(3):88–94.
Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for Prevention of Cardiovascular Disease in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2016;316(19):2008–24.
Beaird SL. HMG-CoA reductase inhibitors: assessing differences in drug interactions and safety profiles. J Am Pharm Assoc (Washington, DC : 1996). 2000;40(5):637–44.
Iwere RB, Hewitt J. Myopathy in older people receiving statin therapy: a systematic review and meta-analysis. Br J Clin Pharmacol. 2015;80(3):363–71.
Kashani A, Phillips CO, Foody JM, Wang Y, Mangalmurti S, Ko DT, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114(25):2788–97.
Riaz H, Khan AR, Khan MS, Rehman KA, Alansari SAR, Gheyath B, et al. Meta-analysis of Placebo-Controlled Randomized Controlled Trials on the Prevalence of Statin Intolerance. Am J Cardiol. 2017;120(5):774–81.
Wong YJ, Qiu TY, Ng GK, Zheng Q, Teo EK. Efficacy and Safety of Statin for Hepatocellular Carcinoma Prevention Among Chronic Liver Disease Patients: A Systematic Review and Meta-analysis. J Clin Gastroenterol. 2021;55(7):615–23.
Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246 955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes. 2013;6(4):390–9.
Ray KK, Cannon CP. Early time to benefit with intensive statin treatment: could it be the pleiotropic effects? Am J Cardiol. 2005;96(5a):54f–60f.
Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21(11):1712–9.
LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352(14):1425–35.
Pedersen TR, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Holme I, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294(19):2437–45.
Rouleau J. Improved outcome after acute coronary syndromes with an intensive versus standard lipid-lowering regimen: results from the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) trial. Am J Med. 2005;118(Suppl 12A):28–35.
Afilalo J, Majdan AA, Eisenberg MJ. Intensive statin therapy in acute coronary syndromes and stable coronary heart disease: a comparative meta-analysis of randomised controlled trials. Heart. 2007;93(8):914–21.
Josan K, Majumdar SR, McAlister FA. The efficacy and safety of intensive statin therapy: a meta-analysis of randomized trials. CMAJ. 2008;178(5):576–84.
Kang S, Liu Y, Liu XB. Effects of aggressive statin therapy on patients with coronary saphenous vein bypass grafts: a systematic review and meta-analysis of randomized, controlled trials. Clin Ther. 2013;35(8):1125–36.
Yan YL, Qiu B, Hu LJ, Jing XD, Liu YJ, Deng SB, et al. Efficacy and safety evaluation of intensive statin therapy in older patients with coronary heart disease: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2013;69(12):2001–9.
Ward NC, Watts GF, Eckel RH. Statin Toxicity. Circ Res. 2019;124(2):328–50.
Hopewell JC, Offer A, Haynes R, Bowman L, Li J, Chen F, et al. Independent risk factors for simvastatin-related myopathy and relevance to different types of muscle symptom. Eur Heart J. 2020;41(35):3336–42.
Ruscica M, Ferri N, Banach M, Sirtori CR, Corsini A. Side effects of statins-from pathophysiology and epidemiology to diagnostic and therapeutic implications. Cardiovasc Res. 2022. https://doi.org/10.1093/cvr/cvac020
Cobos-Palacios L, Sanz-Canovas J, Munoz-Ubeda M, Lopez-Carmona MD, Perez-Belmonte LM, Lopez-Sampalo A, et al. Statin Therapy in Very Old Patients: Lights and Shadows. Front Cardiovasc Med. 2021;8:779044.
Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. Atorvastatin: safety and tolerability. Expert Opin Drug Saf. 2010;9(4):667–74.
Banach M, Rizzo M, Toth PP, Farnier M, Davidson MH, Al-Rasadi K, et al. Statin intolerance - an attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch Med Sci 2015;11(1):1-23.
Mueller AM, Liakoni E, Schneider C, Burkard T, Jick SS, Krähenbühl S, et al. The Risk of Muscular Events Among New Users of Hydrophilic and Lipophilic Statins: an Observational Cohort Study. J Gen Intern Med. 2021;36(9):2639–47.
Keen HI, Krishnarajah J, Bates TR, Watts GF. Statin myopathy: the fly in the ointment for the prevention of cardiovascular disease in the 21st century? Expert Opin Drug Saf. 2014;13(9):1227–39.
Lavan AH, Gallagher P. Predicting risk of adverse drug reactions in older adults. Ther Adv Drug Saf. 2016;7(1):11–22.
Thompson PD, Panza G, Zaleski A, Taylor B. Statin-Associated Side Effects. J Am Coll Cardiol. 2016;67(20):2395–410.
Turner RM, Pirmohamed M. Statin-Related Myotoxicity: A Comprehensive Review of Pharmacokinetic, Pharmacogenomic and Muscle Components. J Clin Med. 2019;9(1):1–37.
McKenney JM. Pharmacologic characteristics of statins. Clin Cardiol. 2003;26(4 Suppl 3):Iii32–8.
Bottorff M, Hansten P. Long-term safety of hepatic hydroxymethyl glutaryl coenzyme A reductase inhibitors: the role of metabolism-monograph for physicians. Arch Intern Med. 2000;160(15):2273–80.
Gruer PJ, Vega JM, Mercuri MF, Dobrinska MR, Tobert JA. Concomitant use of cytochrome P450 3A4 inhibitors and simvastatin. Am J Cardiol. 1999;84(7):811–5.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81901425).
Funding
This work was supported by grants from the National Natural Science Foundation of China (81901425).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data extraction and analysis were performed by Qingtao Hou, Yuqin Chen and Yingxiao Zhang. Methodological supervision of the manuscript was provided by Caishuang Pang. The first draft of the manuscript was written by Qingtao Hou and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 23 kb)
ESM 2
(DOCX 174 kb)
ESM 3
(DOCX 22 kb)
ESM 4
(DOCX 23 kb)

Fig. S1
Online Source 3. Pairwise meta-analyses between statins as a class and control treatment: (a) muscle symptoms, (b) myalgia, (c) myopathy, (d) rhabdomyolysis, (e) CK >10×ULN, (f) discontinuation due to muscle adverse events. CK, creatine kinase; CI, confidence interval; RR, relative risk; ULN, upper limit of normal (PNG 2035 kb)
Fig. S2
Online Source 4. Ranking of different-intensity statins based on probability of CK >10×ULN according to the cumulative ranking area (SUCRA): larger probability, lower incidence of CK >10×ULN. CK, creatine kinase; ULN, upper limit of normal; AH, high-intensity atorvastatin; AM, moderate-intensity atorvastatin; FL, low-intensity fluvastatin; FM, moderate-intensity fluvastatin; PiM, moderate-intensity pitavastatin; PrL, low-intensity pravastatin; PrM, moderate-intensity pravastatin; RH, high-intensity rosuvastatin; RM, moderate-intensity rosuvastatin; SM, moderate-intensity simvastatin (PNG 176 kb)
Fig. S3
Risk-of-bias graph of included trials (PNG 7 kb)
Fig. S4
Risk-of-bias summary of included trials (PNG 67 kb)
Fig. S5
The comparison-adjusted funnel plots for the network of muscle adverse events: (a) muscle symptoms, (b) myalgia, (c) myopathy, (d) rhabdomyolysis, (e) CK >10×ULN, (f) discontinuation due to muscle adverse events. CK, creatine kinase; ULN, upper limit of normal (PNG 1445 kb)
Fig. S6
Loop-specific inconsistency approach for muscle adverse events: (a) muscle symptoms, (b) myalgia, (c) myopathy, (d) rhabdomyolysis, (e) CK >10×ULN, (f) discontinuation due to muscle adverse events. CK, creatine kinase; CI, confidence interval; IF, inconsistency factors; ULN, upper limit of normal (PNG 1197 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hou, Q., Chen, Y., Zhang, Y. et al. Comparative Muscle Tolerability of Different Types and Intensities of Statins: A Network Meta-Analysis of Double-Blind Randomized Controlled Trials. Cardiovasc Drugs Ther (2022). https://doi.org/10.1007/s10557-022-07405-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s10557-022-07405-0