Abstract
Purpose
Inflammation plays an important role in the initiation and progression of atherosclerosis, leading to poor clinical outcomes. Hyperuricemia is associated with the activation of the Nod-like receptor protein 3 inflammasome. Here, we investigated whether inhibition of inflammation using febuxostat lowered the risk of cardiovascular events.
Methods
This is a post-hoc analysis of the randomized trial, Febuxostat for Cerebral and CaRdiorenovascular Events PrEvEntion StuDy (FREED). In total, 1067 patients (736 men and 331 women) were included in the analysis. We compared the serial changes in high-sensitivity C-reactive protein (hs-CRP) levels between febuxostat and non-febuxostat groups and assessed the correlation between the changes in uric acid (UA) and hs-CRP levels after febuxostat treatment. We also determined whether febuxostat could reduce a hard endpoint, defined as a composite of cardiovascular events and all-cause mortality.
Results
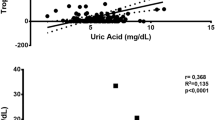
Serum UA levels in the febuxostat group were significantly lower than those in the non-febuxostat group after randomization (p < 0.05). However, hs-CRP levels were comparable between the two groups during the study. No significant correlation was observed between the changes in UA and hs-CRP levels after febuxostat treatment. The hard endpoints did not differ significantly between the two groups. In patients with baseline hs-CRP levels > 0.2 mg/dL or those administered 40 mg of febuxostat, the drug did not reduce hs-CRP levels or decrease the hard endpoint.
Conclusion
Febuxostat reduced the UA levels but did not affect the CRP levels, and therefore may fail to improve cardiovascular outcomes after treatment.
Trial Registration
ClinicalTrial.gov (NCT01984749). https://clinicaltrials.gov/ct2/show/NCT01984749





Similar content being viewed by others
Data Availability
The data underlying this article cannot be shared publicly because the privacy of the individuals who participated in the study must be protected. The data will be shared upon reasonable request to the corresponding author.
References
Wu XW, Muzny DM, Lee CC, Caskey CT. Two independent mutational events in the loss of urate oxidase during hominoid evolution. J Mol Evol. 1992;34:78–84.
Hediger MA, Johnson RJ, Miyazaki H, Endou H. Molecular physiology of urate transport. Physiology (Bethesda). 2005;20:125–33.
Borghi C, Rosei EA, Bardin T, et al. Serum uric acid and the risk of cardiovascular and renal disease. J Hypertens. 2015;33:1729–41.
Johnson RJ, Bakris GL, Borghi C, et al. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the National Kidney Foundation. Am J Kidney Dis. 2018;71:851–65.
Kanbay M, Segal M, Afsar B, Kang DH, Rodriguez-Iturbe B, Johnson RJ. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart. 2013;99:759–66.
Mallat SG, Al Kattar S, Tanios BY, Jurjus A. Hyperuricemia, hypertension, and chronic kidney disease: an emerging association. Curr Hypertens Rep. 2016;18:74.
Neogi T, George J, Rekhraj S, Struthers AD, Choi H, Terkeltaub RA. Are either or both hyperuricemia and xanthine oxidase directly toxic to the vasculature? A critical appraisal. Arthritis Rheum. 2012;64:327–38.
Rentoukas E, Tsarouhas K, Tsitsimpikou C, Lazaros G, Deftereos S, Vavetsi S. The prognostic impact of allopurinol in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Int J Cardiol. 2010;145:257–8.
Grimaldi-Bensouda L, Alpérovitch A, Aubrun E, et al. Impact of allopurinol on risk of myocardial infarction. Ann Rheum Dis. 2015;74:836–42.
Aday AW, Ridker PM. Targeting residual inflammatory risk: a shifting paradigm for atherosclerotic disease. Front Cardiovasc Med. 2019;6:16.
Ridker PM, Everett BM, Thuren T, et al. Anti-inflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–31.
Cabău G, Crișan TO, Klück V, Popp RA, Joosten LAB. Urate-induced immune programming: consequences for gouty arthritis and hyperuricemia. Immunol Rev. 2020;294:92–105.
Kanellis J, Watanabe S, Li JH, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–93.
Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: Implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553–62.
Luis-Rodríguez D, Donate-Correa J, Martín-Núñez E, et al. Serum urate is related to subclinical inflammation in asymptomatic hyperuricaemia. Rheumatology (Oxf Engl). 2021;60:371–9.
Ives A, Nomura J, Martinon F, et al. Xanthine oxidoreductase regulates macrophage IL1-beta secretion upon NLRP3 inflammasome activation. Nat Commun. 2015;6:6555.
Nomura J, Kobayashi T, So A, Busso N. Febuxostat, a xanthine oxidoreductase inhibitor, decreases NLRP3-dependent inflammation in macrophages by activating the purine salvage pathway and restoring cellular bioenergetics. Sci Rep. 2019;9:1–10.
Kojima S, Matsui K, Hiramitsu S, et al. Febuxostat for cerebral and CaRdiorenovascular events PrEvEntion StuDy. Eur Heart J. 2019;40:1778–86.
World Health Organization & International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. World Health Organization. 2006. https://apps.who.int/iris/handle/10665/43588. Accessed 1 May 2022.
Kojima S, Matsui K, Ogawa H, et al. Rationale, design, and baseline characteristics of a study to evaluate the effect of febuxostat in preventing cerebral, cardiovascular, and renal events in patients with hyperuricemia. J Cardiol. 2017;69:169–75.
Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76:29–42.
FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Rheumatol. 2020;72:879–95.
Tardif JC, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–505.
Sethwala AM, Goh I, Amerena JV. Combating inflammation in cardiovascular disease. Heart Lung Circ. 2021;30:197–206.
Kimura K, Hosoya T, Uchida S, et al. Febuxostat therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am J Kidney Dis. 2018;72:798–810.
Tanaka A, Taguchi I, Teragawa H, et al. Febuxostat does not delay progression of carotid atherosclerosis in patients with asymptomatic hyperuricemia: a randomized, controlled trial. PLoS Med. 2020;17:e1003095.
Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328.
Braga TT, Forni MF, Correa-Costa M, et al. Soluble uric acid activates the NLRP3 inflammasome. Sci Rep. 2017;7:39884.
Acknowledgements
We acknowledge the support and assistance of all participating hospitals and patients. We also thank Maki Takami for secretarial assistance.
Funding
The FREED was funded by a grant from Teijin Pharma Ltd. to the Kumamoto Circulation Society according to a support contract. The sponsor was not involved in the planning, implementation, analysis, or interpretation of study results. The first author, corresponding author, and academic statistician had full access to the trial databases and generated trial analyses, prepared the first draft of the manuscript, and decided to submit the manuscript for publication.
Author information
Authors and Affiliations
Consortia
Contributions
Conceptualization, data curation, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing–original draft, writing–review, and editing: S.K.
Investigation, review, and editing: K.U., N.Y., E.T., Y.W., S.H., M.W., H.J., H.K., T.H., N.K., M.S., and H.M.
Project administration, resources, writing (review and editing): K.T.
Conceptualization, formal analysis, methodology, software, validation, review, and editing: K.M.
Conceptualization, investigation, supervision, validation, visualization, reviewing, and editing: I.H., Y.O., K.K., and Y.S.
Conceptualization, funding acquisition, investigation, methodology, supervision, validation, visualization, review, and editing: H.O.
Corresponding author
Ethics declarations
Ethical Approval
FREED was a multicenter, prospective, randomized, open-label, blinded endpoint, two-arm parallel treatment group study conducted as an investigator-initiated study following the principles of the Declaration of Helsinki and the Ethical Guidelines for Clinical Studies issued by the Ministry of Health, Labour, and Welfare in Japan. The FREED protocol was reviewed by the central institutional review board before approval by the institutional review board of each participating study site, and all the patients registered with the FREED provided written informed consent. The FREED is registered at ClinicalTrial.gov (identification number: NCT01984749).
Consent to Participate
All the patients registered with the FREED provided written informed consent. In this post-hoc analysis, 1070 patients from the FREED were analyzed, and the requirement for informed consent was waived.
Conflicts of Interest
S.K. reports personal fees from Teijin Healthcare, limited, personal fees from Teijin Pharma and Otsuka Pharmaceutical Co., Ltd., personal fees from Mochida Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., and Novartis K.K., and grants from Grants-in-Aid for Scientific Research (21K07356) of the Japan Society for the Promotion of Science outside the submitted work. K.T. reports and has received remuneration for lectures at Amgen K.K., Bayer Yakuhin, Ltd., Daiichi Sankyo Co., Ltd., Kowa Pharmaceutical Co., Ltd., Novartis Pharma K.K., Otsuka Pharmaceutical Co., Ltd., and Pfizer Japan Inc., trust research/joint research funds from AMI Co., Ltd., Bayer Yakuhin, Ltd., Bristol-Myers K.K., EA Pharma Co., Ltd., and Mochida Pharmaceutical Co., Ltd., scholarship funds from AMI Co., Ltd., Bayer Yakuhin, Ltd., Boehringer Ingelheim Japan, Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Edwards Lifesciences Corporation, Johnson & Johnson K.K., Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd., and affiliations with endowed departments from Abbott Japan Co., Ltd., Boston Scientific Japan K.K., Fides-one, Inc., GM Medical Co., Ltd., ITI Co., Ltd., Kaneka Medix Co., Ltd., Nipro Corporation, Terumo Co., Ltd., Abbott Medical Co., Ltd., Boston Scientific Japan K.K., Cardinal Health Japan, Fukuda Denshi Co., Ltd., Japan Lifeline Co., Ltd., Medical Appliance Co., Ltd., and Medtronic Japan Co., Ltd. outside the submitted work. I.H. reports grants and personal fees from Sanwa Kagaku Kenkyusho Co., Ltd., Fuji Yakuhin Co., Ltd., personal fees from Pfizer Co., Ltd., and grants from Dainippon Sumitomo Pharma Co., Ltd., and Teijin Pharma outside the submitted work. K.K. reports grants from Teijin Pharma and personal fees from Tanabe Mitsubishi outside the submitted work. Y.S. reports grants from MEXT KAKENHI (grant number JP19155855), Health Labour Sciences Research (grant number 19189094), Health Labour Sciences Research (grant number 17933459), AMED (grant numbers JP19ek0210080, JP19ek0210118, JP19ek0210121, JP19ek0210115, JP19ek0109367, JP19ek0109406, and JP19km0405009), Takeda Pharmaceutical Co., Ltd., Actelion Pharmaceuticals Japan Ltd., Kyowa Kirin Co., Ltd., Shionogi & Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Nihon Medi-Physics Co., Ltd., and Fuji Yakuhin Co., Ltd. during the study, grants and personal fees from Otsuka Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Bristol-Myers Squibb Company, Kowa Pharmaceutical Co., Ltd., Teijin Pharma Ltd., and Pfizer Japan Inc., grants, personal fees, and other from Novartis Pharma K.K. and Bayer Yakuhin, Ltd., other from Amgen Astellas BioPharma K.K., Actelion Pharmaceuticals Japan Ltd., and Roche Diagnostics K.K., personal fees from Alnylam Japan K.K., AstraZeneca K.K., Tsumura & Co., Toa Eiyo Ltd., Nippon Shinyaku Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., and Mochida Pharmaceutical Co., Ltd. outside the submitted work. H.O. reports personal fees from Towa Pharmaceutical and Novartis Pharma outside the submitted work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 489 kb)
Rights and permissions
About this article
Cite this article
Kojima, S., Uchiyama, K., Yokota, N. et al. C-reactive Protein Levels and Cardiovascular Outcomes After Febuxostat Treatment in Patients with Asymptomatic Hyperuricemia: Post-hoc Analysis of a Randomized Controlled Study. Cardiovasc Drugs Ther 37, 965–974 (2023). https://doi.org/10.1007/s10557-022-07347-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-022-07347-7