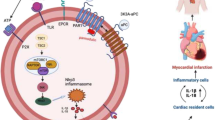
Abstract
Purpose
Recombinant apyrase (AZD3366) increases adenosine production and ticagrelor inhibits adenosine reuptake. We investigated whether intravenous AZD3366 before reperfusion reduces myocardial infarct size (IS) and whether AZD3366 and ticagrelor have additive effects.
Methods
Sprague–Dawley rats underwent 30 min ischemia. At 25 min of ischemia, animals received intravenous AZD3366 or vehicle. Additional animals received intravenous CGS15943 (an adenosine receptor blocker) or intraperitoneal ticagrelor. At 24 h reperfusion, IS was assessed by triphenyltetrazolium chloride. Other rats were subjected to 30 min ischemia followed by 1 h or 24 h reperfusion. Myocardial samples were assessed for adenosine levels, RT-PCR, and immunoblotting.
Results
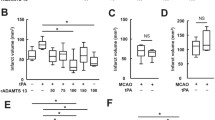
AZD3366 and ticagrelor reduced IS. The protective effect was blocked by CGS15943. The effect of AZD3366 + ticagrelor was significantly greater than AZD3366. One hour after infarction, myocardial adenosine levels significantly increased with AZD3366, but not with ticagrelor. In contrast, 24 h after infarction, adenosine levels were equally increased by AZD3366 and ticagrelor, and levels were higher in the AZD3366 + ticagrelor group. One hour after reperfusion, AZD3366 and ticagrelor equally attenuated the increase in interleukin-15 (an early inflammatory marker after ischemic cell death) levels, and their combined effects were additive. AZD3366, but not ticagrelor, significantly attenuated the increase in RIP1, RIP3, and P-MLKL (markers of necroptosis) 1 h after reperfusion. AZD3366, but not ticagrelor, significantly attenuated the increase in IL-6 and GSDMD-N (markers of pyroptosis) 1 h after reperfusion. At 24 h of reperfusion, both agents equally attenuated the increase in these markers, and their effects were additive.
Conclusions
AZD3366 attenuated inflammation, necrosis, necroptosis, and pyroptosis and limited IS. The effects of AZD3366 and ticagrelor were additive.











Similar content being viewed by others
Availability of Data and Material
Original research data will be available upon request.
Code Availability
Not applicable.
References
Forman MB, Stone GW, Jackson EK. Role of adenosine as adjunctive therapy in acute myocardial infarction. Cardiovasc Drug Rev. 2006;24(2):116–47.
Maurer G. Adenosine as an adjunct to reperfusion in myocardial infarction. Eur Heart J. 2006;27(20):2376–7.
Bulluck H, Sirker A, Loke YK, Garcia-Dorado D, Hausenloy DJ. Clinical benefit of adenosine as an adjunct to reperfusion in ST-elevation myocardial infarction patients: an updated meta-analysis of randomized controlled trials. Int J Cardiol. 2016;202:228–37.
Manickavasagam S, Ye Y, Lin Y, et al. The cardioprotective effect of a statin and cilostazol combination: relationship to Akt and endothelial nitric oxide synthase activation. Cardiovasc Drugs Ther. 2007;21(5):321–30.
Merla R, Ye Y, Lin Y, et al. The central role of adenosine in statin-induced ERK1/2, Akt, and eNOS phosphorylation. Am J Physiol Heart Circ Physiol. 2007;293(3):H1918–28.
Ye Y, Abu Said GH, Lin Y, et al. Caffeinated coffee blunts the myocardial protective effects of statins against ischemia-reperfusion injury in the rat. Cardiovasc Drugs Ther. 2008;22(4):275–82.
Birnbaum Y, Birnbaum GD, Birnbaum I, Nylander S, Ye Y. Ticagrelor and rosuvastatin have additive cardioprotective effects via adenosine. Cardiovasc Drugs Ther. 2016;30(6):539–50.
Ye Y, Birnbaum GD, Perez-Polo JR, et al. Ticagrelor protects the heart against reperfusion injury and improves remodeling after myocardial infarction. Arterioscler Thromb Vasc Biol. 2015;35(8):1805–14.
van der Pals J, Koul S, Gotberg MI, et al. Apyrase treatment of myocardial infarction according to a clinically applicable protocol fails to reduce myocardial injury in a porcine model. BMC Cardiovasc Disord. 2010;10:1.
Kaczmarek E, Koziak K, Sevigny J, et al. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996;271(51):33116–22.
Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5’-nucleotidase (CD73). Purinergic Signal. 2006;2(2):351–60.
Armstrong D, Summers C, Ewart L, et al. Characterization of the adenosine pharmacology of ticagrelor reveals therapeutically relevant inhibition of equilibrative nucleoside transporter 1. J Cardiovasc Pharmacol Ther. 2014;19(2):209–19.
Smith SB, Xu Z, Novitskaya T, et al. Impact of cardiac-specific expression of CD39 on myocardial infarct size in mice. Life Sci. 2017;179:54–9.
Moeckel D, Jeong SS, Sun X et al. Optimizing human apyrase to treat arterial thrombosis and limit reperfusion injury without increasing bleeding risk. Sci Transl Med. 2014;6(248):248ra105.
Wheeler DG, Joseph ME, Mahamud SD, et al. Transgenic swine: expression of human CD39 protects against myocardial injury. J Mol Cell Cardiol. 2012;52(5):958–61.
Kohler D, Eckle T, Faigle M, et al. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 2007;116(16):1784–94.
Xu Z, Chen W, Zhang R, et al. Human recombinant apyrase therapy protects against myocardial ischemia/reperfusion injury and preserves left ventricular systolic function in rats, as evaluated by 7T cardiovascular magnetic resonance imaging. Korean J Radiol. 2020;21(6):647–59.
Nanhwan MK, Ling S, Kodakandla M, et al. Chronic treatment with ticagrelor limits myocardial infarct size: an adenosine and cyclooxygenase-2-dependent effect. Arterioscler Thromb Vasc Biol. 2014;34(9):2078–85.
Wojcik WJ, Neff NH. Adenosine measurement by a rapid HPLC-fluorometric method: induced changes of adenosine content in regions of rat brain. J Neurochem. 1982;39(1):280–2.
Birnbaum Y, Ye Y, Lin Y, et al. Aspirin augments 15-epi-lipoxin A4 production by lipopolysaccharide, but blocks the pioglitazone and atorvastatin induction of 15-epi-lipoxin A4 in the rat heart. Prostaglandins Other Lipid Mediat. 2007;83(1–2):89–98.
Ye Y, Martinez JD, Perez-Polo RJ, et al. The role of eNOS, iNOS, and NF-kappaB in upregulation and activation of cyclooxygenase-2 and infarct size reduction by atorvastatin. Am J Physiol Heart Circ Physiol. 2008;295(1):H343–51.
Chen H, Tran D, Yang HC, et al. Dapagliflozin and ticagrelor have additive effects on the attenuation of the activation of the NLRP3 inflammasome and the progression of diabetic cardiomyopathy: an AMPK-mTOR interplay. Cardiovasc Drugs Ther. 2020;34(4):443–61.
Ye Y, Bajaj M, Yang HC, Perez-Polo JR, Birnbaum Y. SGLT-2 inhibition with dapagliflozin reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic cardiomyopathy in mice with type 2 diabetes. Further Augmentation of the Effects with Saxagliptin, a DPP4 Inhibitor. Cardiovasc Drugs Ther. 2017;31(2):119–32.
Turillazzi E, Di Paolo M, Neri M, Riezzo I, Fineschi V. A theoretical timeline for myocardial infarction: immunohistochemical evaluation and western blot quantification for Interleukin-15 and Monocyte chemotactic protein-1 as very early markers. J Transl Med. 2014;12:188.
Kubica J, Adamski P, Ostrowska M, et al. Influence of morphine on pharmacokinetics and pharmacodynamics of ticagrelor in patients with acute myocardial infarction (IMPRESSION): study protocol for a randomized controlled trial. Trials. 2015;16:198.
Parodi G, Bellandi B, Xanthopoulou I et al. Morphine is associated with a delayed activity of oral antiplatelet agents in patients with ST-elevation acute myocardial infarction undergoing primary percutaneous coronary intervention. Circ Cardiovasc Interv. 2015;8(1)
Silvain J, Storey RF, Cayla G, et al. P2Y12 receptor inhibition and effect of morphine in patients undergoing primary PCI for ST-segment elevation myocardial infarction. The PRIVATE-ATLANTIC study. Thromb Haemost. 2016; 116(2):369–78.
Montalescot G, van 't Hof AW, Lapostolle F et al. Prehospital ticagrelor in ST-segment elevation myocardial infarction. N Engl J Med. 2014;371(11):1016–27.
Montalescot G, van 't Hof AW, Bolognese L et al. Effect of pre-hospital ticagrelor during the first 24 h after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction: the ATLANTIC-H(2)(4) analysis. JACC Cardiovasc Interv. 2016;9(7):646–56.
Mishra PK, Adameova A, Hill JA, et al. Guidelines for evaluating myocardial cell death. Am J Physiol Heart Circ Physiol. 2019;317(5):H891–922.
Heusch G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol. 2020;17(12):773–89.
Toldo S, Mauro AG, Cutter Z, Abbate A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2018;315(6):H1553–68.
Ridker PM, Rane M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ Res. 2021;128(11):1728–46.
Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004;61(3):448–60.
Funding
The study was funded by an investigator-initiated grant from AstraZeneca and the John S. Dunn Chair in Cardiology Research and Education.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, experiments, and data collection were performed by Yumei Ye and Huan Chen. Data analysis was performed by Yumei Ye and Yochai Birnbaum. Figures were made by Yochai Birnbaum. The first draft of the manuscript was written by Yochai Birnbaum and Regina Ye, and all authors read and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The experimental designs and animal care were approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch.
Human and Animals Rights and Informed Consent
The experimental designs and animal care were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Consent to participate
Not applicable.
Consent for publication
All authors read and approved the final manuscript and submitted for consideration of publication in Basic Research in Cardiology.
Conflict of interest
Yochai Birnbaum: Research grant from AstraZeneca. Regina Ye: None. Huan Chen: None. Leif Carlsson is a former employee of AstraZeneca and holds stocks in AstraZeneca. Carl Whatling is an employee of Astra Zeneca. Ola Fjellström is an employee of Astra Zeneca. Erik Ryberg is an employee of Astra Zeneca. Yumei Ye: Research grant from AstraZeneca.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Birnbaum, Y., Ye, R., Chen, H. et al. Recombinant Apyrase (AZD3366) Against Myocardial Reperfusion Injury. Cardiovasc Drugs Ther 37, 625–646 (2023). https://doi.org/10.1007/s10557-022-07329-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-022-07329-9