Abstract
Recent studies of the human microbiome have offered new insights into how the microbiome can impact cancer development and treatment. Specifically, in pancreatic ductal adenocarcinoma (PDAC), the microbiota has been shown to modulate PDAC risk, contribute to tumorigenesis, impact the tumor microenvironment, and alter treatment response. These findings provide rationale for further investigations into leveraging the microbiome to develop new strategies to diagnose and treat PDAC patients. There is growing evidence that microbiome analyses have the potential to become easily performed, non-invasive diagnostic, prognostic, and predictive biomarkers in pancreatic cancer. More excitingly, there is now emerging interest in developing interventions based on the modulation of microbiota. Fecal microbiota transplantation, probiotics, dietary changes, and antibiotics are all potential strategies to augment the efficacy of current therapeutics and reduce toxicities. While there are still challenges to overcome, this is a rapidly growing field that holds promise for translation into clinical practice and provides a new approach to improving patient outcomes.
Similar content being viewed by others
1 Introduction
Pancreatic ductal adenocarcinoma (PDAC) accounts for more than 90% of all pancreatic cancers and is the third leading cause of cancer-related deaths in the USA [1]. An estimated 60,430 new cases of PDAC will be diagnosed in 2021 [1]. Surgical resection remains the only curative therapy but only around 20% of patients have resectable tumors at diagnosis [2]. Most patients with PDAC present with either locally advanced (40%) or metastatic (40%) disease, and standard-of-care treatments are limited to palliative systemic therapies. Despite continued efforts, the 5-year overall survival rate of PDAC patients remains around 10% for all stages combined and drops to only 3% for those with distant disease [3]. Multiple factors contribute to the poor outcomes including nonspecific symptoms, the lack of early diagnostic markers, aggressive tumor biology/early metastasis, and resistance to chemotherapy. Clearly, there is a critical need for novel approaches to screening, prevention, and treatment.
The microbiota (bacteria, archaea, viruses, fungi, protozoa) inhabiting the human body are estimated to be in the range of 10–100 trillion [4]. The combined genetic material harbored by these microorganisms, known as the microbiome, far exceeds that of the human genome [5]. While the majority of microorganisms reside in the gastrointestinal tracts, microbiota can be found in other external and internal surfaces of the human body such as the skin, oral cavity, conjunctiva, and genitourinary tracts [6]. They play an essential role in maintaining homeostasis in the human body, and an imbalance of the microbiota, a state known as dysbiosis, can contribute to the pathogenesis of many diseases [7]. Increasingly, the microbiota is recognized to contribute to carcinogenesis and treatment outcomes [8]. Microbes such as Helicobacter pylori, human papillomaviruses, and hepatitis viruses have been linked to human malignancies, and infectious agents are thought to underlie the development of 10–20% of the global cancer cases each year [9]. Microbial dysbiosis can also positively and negatively impact tumor responses to therapies [10]. The microbiota has the ability to promote inflammatory responses, change the tumor immune microenvironment, alter tumor metabolism, and modulate tumor sensitivity to drugs [11,12,13,14,15]. Thus, the microbiome holds significant promise when developing PDAC management strategies.
In this review, we will summarize the current understanding on the role of bacteria and fungi in PDAC tumorigenesis and explore the potential translational implications of targeting the microbiome in the management of PDAC.
2 The microbiome and pancreatic cancer
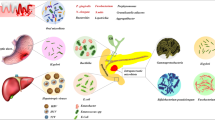
While researchers first noted the presence of bacteria in human tumors more than 100 years ago, these microbes were difficult to characterize due to their low biomass and the possibility of tumor sample contamination [16]. The development of newer analysis techniques has allowed for more in-depth examination and characterization of these microbes in recent years. The most common technique used to identify bacterial populations is the sequencing of amplified 16S ribosomal RNA (rRNA), and a similar technique that relies on the sequencing of amplified internal transcriber spacer (ITS) regions between various rRNA subunit genes can be used to identify the fungal composition in samples [17, 18]. Using these techniques, researchers have been able to interrogate the microbiomes of both healthy individuals and PDAC patients, with interesting patterns emerging (Figure 1).
Microbiota in pancreatic cancer. Created with BioRender.com
2.1 Microbiota in pancreas
Historically, the pancreas was thought to be a sterile organ, but recent studies have found the existence of bacteria populations in both normal pancreatic tissue and PDAC tumor samples. Nejman et al. examined the bacterial composition of 1010 tumor samples across 7 different tumor types and 516 normal samples [16]. They found that pancreatic cancer had one of the highest proportions of tumor positive for bacterial DNA, and bacteria belonging to the Proteobacteria phylum were most abundant [16]. Geller et al. conducted real-time quantitative polymerase chain reaction (qPCR) to detect bacterial 16S ribosomal DNA in 113 human PDAC samples and 20 normal pancreas samples [15]. They were able to detect bacterial DNA in 15% of the normal pancreas samples and 76% of the PDAC samples, and the presence of intratumoral bacteria was confirmed by fluorescence in situ hybridization (FISH) targeted to 16S rRNA and immunohistochemistry. The authors further examined the specific bacterial species present in the tumors by sequencing amplified 16S rRNA genes and found that the most common species belonged to the Enterobacteriaceae and Pseudomonadaceae families [15]. Pushalkar et al. also compared the bacteria composition of 12 PDAC samples and 5 normal pancreas samples [19]. By using FISH, the authors found that the human PDAC samples harbored higher abundance of bacteria when compared to the normal pancreas samples. Sequencing of 16S rRNA revealed that the most prevalent intratumoral bacteria phyla included Proteobacteria, Bacteroidetes, and Firmicutes. Assessment of clade abundances confirmed that the bacterial composition of PDAC was distinct from normal human pancreas [19]. Additional studies of the microbiota in PDAC tissue also confirmed the presence of a distinct bacterial intratumoral profile [16, 20, 21].
Similar to the bacterial findings, Aykut et al. recently showed that the fungal flora (mycobiome) of PDAC samples are distinct from healthy samples as well [22]. They analyzed the intrapancreatic mycobiome of 13 PDAC patients and 5 healthy individuals, and they discovered that the fungal communities of PDAC samples clustered separately from the healthy samples. Specifically, PDAC samples were enriched for Malassezia species [22].
2.2 Gastrointestinal microbiota
In addition to intratumoral dysbiosis, several studies have shown a difference in the gut microbiota between PDAC patients and healthy individuals. In a study by Ren et al., fecal samples from 85 PDAC patients and 57 matched healthy controls were collected prospectively and analyzed for their microbial characteristics [23]. The gut microbial diversity was found to be significantly lower in PDAC patients. The composition of the gut microbiota was also unique in PDAC patients and contained significantly higher Bacteroidetes and lower Firmicutes and Proteobacteria when compared to healthy controls [23]. Rogers et al. characterized the bacterial composition of fecal samples, pancreatic fluid, bile, and jejunal fluid from 50 patients undergoing pancreaticoduodenectomy [21]. The microbial diversity of the fecal samples was significantly lower than samples from healthy volunteers, and they were enriched with Klebsiella and Bacteroides [21]. A separate study conducted by Half and colleagues examined the fecal microbiota of 30 PDAC patients, 13 healthy individuals, and 16 patients with non-alcoholic fatty liver disease [24]. While this study did not find any differences in the microbial diversity between groups, results did show that PDAC patients had a distinct microbial profile when compared to controls and had a higher Bacteroidetes to Firmicutes ratio, which is consistent with the findings of Ren et al. [23, 24].
In a small case-control study, analysis of the duodenal mucosal microbiota in 14 PDAC patients and 14 healthy individuals showed no significant difference between the two groups [25]. However, a later study of bacterial and fungal profiles of duodenal fluid from 74 PDAC patients, 98 patients with pancreatic cysts, and 134 normal controls revealed that patients with PDAC had significantly decreased bacterial and fungal diversity when compared to patients with pancreatic cysts and healthy controls [26]. PDAC patients also had an enrichment of Bifidobacterium and Ascomycota compared to healthy individuals. The duodenal fluid microbiota profiles were not significantly different between patients with pancreatic cysts and healthy controls [26].
The differences in study sizes, designs, sampling methods, and primers used for 16S rRNA amplification make interpretation and generalization difficult. Moreover, the decrease in gut microbial diversity compared to healthy individuals is observed in other chronic diseases as well [27,28,29].
2.3 Oral microbiota
Finally, the oral microbiota is different between PDAC patients and healthy controls as well. Lu and colleagues examined the tongue coating microbiota in 30 PDAC patients and 25 healthy controls [30]. They found that the microbiota diversity of the tongue coat in PDAC patients was significantly increased and the bacterial composition of the tongue coating is markedly different between PDAC patients and controls. A few bacterial genera (Haemophilus, Porphyromonas, Leptotrichia, and Fusobacterium) could distinguish PDAC patients from healthy individuals [30].
Additionally, multiple studies have shown a difference between the saliva microbiota profiles of PDAC patients compared to healthy controls, though different bacteria species were identified as the distinguishing factor in the different studies [31,32,33,34,35]. Similar to the gut microbiota studies, the inconsistency may be related to differences in study size, study design, and geographic location.
2.4 Microbial dysbiosis and PDAC risk
Prior epidemiologic studies have identified multiple risk factors for the development of PDAC including age, family history, cigarette smoking, obesity, chronic pancreatitis, and type 2 diabetes [36,37,38,39,40,41]. Microbial dysbiosis is associated with many of these environmental risk factors such obesity, chronic pancreatitis, and type 2 diabetes, which can potentially impact the risk of PDAC [42,43,44,45,46,47,48].
Interestingly, periodontal disease has been linked to increased PDAC risk. Hujoel et al. analyzed data from 11,328 patients from the National Health and Nutrition Examination Survey (NHANES) I Epidemiologic Follow-up Study and found a positive but nonsignificant association between periodontitis and PDAC risk (odds ratio [OR], 1.77; 95% confidence interval [CI], 0.85–3.67) [49]. In another prospective cohort study that included 48,375 US male health professionals, Michaud et al. found that after adjusting for known risk factors such as smoking history, body mass index, and history of diabetes, patients with a history of periodontal disease had significantly increased risk for PDAC (hazard ratio [HR], 1.54; 95% CI, 1.16–2.04) [50]. In a third cohort study, Chang et al. examined data from 139,805 patients and reported a significantly positive association between periodontal disease and PDAC risk (HR, 1.55; 95% CI, 1.02–2.33) [51]. This association is independent of age, sex, and medical comorbidities including diabetes, hyperlipidemia, allergies, viral hepatitis, peptic ulcer, pancreatitis, chronic obstructive pulmonary disease (as a proxy for cigarette smoking), and alcoholic-related conditions (as a proxy for alcohol drinking) [51]. Finally, a meta-analysis of 8 studies by Maisonneuve and colleagues confirmed a significantly positive association between periodontitis and PDAC risk (relative risk [RR], 1.74; 95% CI, 1.41–2.15) [52].
The increased risk for PDAC from periodontal disease may be related to oral dysbiosis. Oral microbes such as Porphyromonas gingivalis are important contributors to periodontal disease and may cause systemic inflammation leading to carcinogenesis [53, 54]. Michaud et al. conducted a nested case-control study of 405 PDAC patients and 416 healthy controls to examine the relationship between antibodies to oral microbes and risk of PDAC [55]. Results show that higher levels of antibodies against a pathogenic strain of P. gingivalis were associated with a twofold increase in the risk of PDAC (OR, 2.14; 95% CI, 1.05–4.36; >200 ng/ml vs ≤200 ng/ml). Additionally, higher levels of antibodies against commensal (nonpathogenic) oral bacteria were associated with 45% lower risk of PDAC when compared to those with lower levels of antibodies (OR, 0.55; 95% CI, 0.36–0.83), suggesting that certain commensal oral bacteria may counter dysbiosis from pathogenic bacteria growth, thus decreasing the risk for PDAC [55].
The above results suggest that oral dysbiosis may play a role in PDAC development, though they need to be interpreted with caution given that it is difficult to know whether the change in the microbiota led to cancer development or whether the cancer caused the change in the microbiota. This has been explored by Fan et al. in a prospective nested case-control study, albeit not definitively, to assess the relationship of oral microbiota with risk of PDAC [56]. The study included 361 PDAC patients and 371 matched controls, and their pre-diagnostic oral wash samples were evaluated to determine the bacterial composition. The presence of P. gingivalis and Aggregatibacter actinomycetemcomitans, both considered oral pathogens, was associated with higher risk of PDAC (OR, 1.60; 95% CI, 1.15–2.22 for P. gingivalis; OR, 2.20; 95% CI, 1.16–4.18 for A. actinomycetemcomitans), while Fusobacteria was associated with decreased risk of PDAC (OR, 0.94, 95% CI, 0.89–0.99) [56]. These risks remained even after excluding patients who developed pancreatic cancer within 2 years of sample collection, so the likelihood of reverse causation is reduced. Further prospective cohort studies in high-risk patients evaluating the change in the microbiota over time will be needed to clarify this association.
2.5 Potential role of microbiota in pancreas carcinogenesis
While the aforementioned cohort studies linked microbial dysbiosis and PDAC risk, preclinical models have further clarified the potential mechanisms by which microbiota can contribute to tumorigenesis and establish a more causative role. Gnansekaran et al. evaluated the direct effects of P. gingivalis on PDAC development and proliferation using cell lines and a xenograft model [57]. They found that P. gingivalis infection enhanced proliferation of PDAC cells, and the enhanced tumor cell proliferation correlates with P. gingivalis intracellular survival. Hypoxia increased P. gingivalis intracellular survival. Consistent with the in vitro results, the authors found that P. gingivalis infection also led to enhanced tumor growth in vivo. A prior study in an oral squamous cell model indicated that of the effects of P. gingivalis on cancer cell growth was mediated through a Toll-like receptor 2 (TLR2)-dependent mechanism [58]. In contrast, Gnansekaran et al. found that P. gingivalis-induced PDAC cell proliferation was independent of TLR2 signaling and is associated with augmentation of the Akt signaling pathway [57].
Others showed that intestinal microbiota may affect the tumor immune microenvironment and impact PDAC tumor growth and therapy response. By using genetically engineered PDAC mouse models (KC and KPC), Pushalkar et al. showed that intestinal bacteria can migrate into the pancreas, and PDAC samples contained a distinct microbial profile when compared to normal samples [19]. Additionally, they demonstrated that tumor initiation and progression was delayed in both germ-free mice and mice treated with an oral antibiotic regimen to ablate the gut microbiota, and repopulation of the gut microbiota by fecal transplant from PDAC-bearing mice or treatment with Bifidobacterium pseudolongum accelerated disease progression. Microbial ablation altered the tumor microenvironment and resulted in increased M1 macrophage differentiation and tumor infiltration of T cells as well as decreased myeloid-derived suppressor cells. Microbial ablation also improved the antitumor effects of PD-1 blockade by upregulating PD-1 expression, suggesting that the microbiota may be a potential therapeutic target in PDAC [19]. A separate study by Thomas et al. in a different mouse model (KrasG12D/PTENlox/+) showed that antibiotic treatment decreased the incidence of cancer [59]. Microbial depletion of Nod-SCID mice harboring PDAC xenografts resulted in increased time to xenograft formation, smaller tumors, and attenuated growth, and these tumors had higher immune cell infiltration [59]. Sethi et al. demonstrated that antibiotic therapy significantly reduced tumor growth and metastatic burden in a PDAC mouse model, and this was associated with an increase in effector T cells in the tumor microenvironment [60]. Antibiotic treatment did not decrease tumor size in Rag1-KO mice, which lack mature T and B cells, suggesting that the antitumor effect of antibiotics was not a direct cytotoxic effect and required active participation of adaptive immunity [60].
The role of mycobiome in PDAC tumorigenesis was examined by Aykut et al [22]. Amphotericin B-mediated mycobiome ablation resulted in delayed tumor development and growth in PDAC mouse models, and repopulation with Malassezia increased the growth of PDAC tumors. The tumorigenic effect of fungal pathogens was found to be mediated by the complement cascade, which was triggered by the binding of pathogens to mannose-binding lectin (MBL) [22].
Taken together, these animal studies provide evidence that microbial dysbiosis alters the tumor immune microenvironment and can influence PDAC tumor development/progression. These results suggest that the microbiota has the potential to be a therapeutic target, but significant challenges remain. Detailed examination of the microbiota in large cohorts of real-world patients with pancreatic cancer is needed to investigate the specific communities that may contribute positively or negatively to disease development and reconcile contradictory findings between animal and human studies. For example, long-term antibiotic use may actually increase the risk of cancer occurrence in human [61], while microbial depletion delayed tumor growth in mice [19, 59, 60]. Another important observation is that prior antibiotic exposure, but not concurrent antibiotic use, can negatively impact the clinical efficacy of immunotherapy in some non-PDAC tumors [62], though there are conflicting data and this continues to be an area of active debate [63, 64]. Further studies are needed to better understand the dynamic functions of the microbiome and its interactions with our immune system so we can harness the microbiome to optimize therapies.
3 Translational implications
Biomarkers for early detection and diagnosis are urgently needed in PDAC, particularly due to the poor prognosis and potential for early metastasis. Recent studies have demonstrated that microbiome analyses have the potential to become non-invasive diagnostic, prognostic, and predictive biomarkers in the management of PDAC.
3.1 Microbiome for early detection/diagnosis of PDAC
Currently, there is no recommended screening program for pancreatic cancer. Early diagnosis allows prompt (and potentially curative) intervention that may lead to better patient outcomes. Serum tests for carbohydrate antigen 19-9 (CA 19-9) are not optimal for screening due to low positive predictive value in asymptomatic patients [65].
The distinct microbiota found in PDAC patients offers novel opportunities to develop diagnostic/screening biomarkers and the tests may be easily performed in the clinic since salivary and fecal samples will be less invasive and easier to obtain than tissue biopsy from metastatic tumors. Farrell and colleagues first explored the possibility of using salivary microbial profile as a diagnostic biomarker by using the Human Oral Microbe Identification Microarray (HOMIM) to compare the salivary microbiota between 10 PDAC patients and 10 matched controls to identify bacterial candidates [34]. This was then validated in a cohort of 28 PDAC samples, 28 matched controls, and 27 chronic pancreatitis samples. The authors observed a significant difference in the salivary microbiota between PDAC patients and controls. Specifically, levels of Neisseria elongata and Streptococcus mitis were significantly lower in patients with pancreatic cancer, and the combination of these two microbial biomarkers showed a sensitivity of 96.4% and specificity of 82.1% in differentiating PDAC patients from healthy subjects. Furthermore, they compared these biomarkers against a HOMIM profiling study in lung cancer and found that none of the bacterial candidates from the PDAC study was significantly altered in lung cancer [34]. Torres et al. also examined the salivary microbiota of PDAC patients, patients with other diseases, and healthy individuals (8 vs 78 vs 22 patients, respectively) [35]. By using high-throughput sequencing, the authors identified a significantly higher ratio of Leptotrichia to Porphyromonas (LP ratio) in the saliva of PDAC patients when compared to the other 2 groups. They thus proposed that the LP ratio may be a potential PDAC diagnostic biomarker. Interestingly, the relative abundances of Neisseria and Streptococcus were not significantly different between the patient groups in this study, and the authors attributed this to a difference in study methodology when compared to the study conducted by Farrell et al [35]. Mendez et al. approached early detection of PDAC by assessing the gut microbiota and its metabolic products in both KPC mice and PDAC patients [66]. They found that the bacterial phyla Proteobacteria and Firmicutes were enriched in the gut microbiota in early PDAC development. This was associated with increased serum polyamine levels as a product of active metabolic pathways. Thus, analysis of metabolites of microbial dysbiosis may be another non-invasive biomarker for early detection of PDAC [66].
Although these studies have identified potential strategies for developing diagnostic biomarkers based on the microbiota, many challenges still exist. One of the major barriers is the variability in the bacterial populations identified across these studies. While some bacterial phyla such as Proteobacteria, Bacteroidetes, and Firmicutes have been found to be enriched in PDAC patients in multiple studies, few studies have identified common genera or species of bacteria that are consistently altered in PDAC. Differences in host ethnicity, geographic location, lifestyle, and dietary intake can lead to divergent baseline microbiome composition, which likely contributes to the variability seen across studies [67,68,69,70,71]. This makes selecting a generalizable biomarker difficult. Thus, further studies will be needed to validate these analytic strategies and identify universal biomarkers for the clinic.
3.2 Microbiome as prognostic biomarker for PDAC
Accurate prognostic biomarkers are important for stratifying treatment strategies according to the biology of PDAC in patients. Several groups have demonstrated the potential of microbiota profile in prognosticating outcomes of PDAC patients.
In a study by Mitsuhashi et al., 283 PDAC tumor samples were tested for Fusobacterium species [72]. The samples were also tested for specific mutations, microRNA expression, and epigenetic alterations. Fusobacterium species were detected in 8.8% of the tumor samples, and Fusobacterium species positivity was associated with significantly shorter cancer-specific survival independent of other clinical and molecular features, suggesting that intratumoral Fusobacterium status may be a potential prognostic biomarker [72]. Kohi et al. examined the bacterial composition of duodenal fluid collections from 74 PDAC patients and compared the microbial profiles of short-term vs long-term survivors [26]. Samples from short-term survivors were enriched with Fusobacterium, Rothia, and Neisseria [26]. In a retrospective study of intraoperative bile cultures from 211 locally advanced or borderline resectable PDAC patients, higher numbers of microbial species were associated with shorter progression free survival (PFS) [73].
However, not all intratumoral bacterial colonization is associated with worse outcomes. Riquelme et al. examined the intratumoral microbiome composition of 68 resected PDAC tumors; 36 of these patients were considered long-term survivors (> 5 years) and 32 were considered short-term survivors (<5 years) [74]. They found that the intratumoral microbiome diversity was significantly higher in the long-term survivors compared with the short-term survivors. A specific microbiome signature (Pseudoxanthomonas, Streptomyces, Saccharopolyspora, Bacillus clausii) was highly predictive of long-term survival [74].
Together, these results suggest that the microbiota diversity and composition of PDAC patients can potentially provide prognostic information to predict the survival of these patients. Once again, further studies are needed to clarify discrepant results and better define the biomarkers.
3.3 Microbiome as a predictive biomarker for treatment response in PDAC
Finally, biomarkers that predict patient response to therapies will be crucial to improve the outcomes of PDAC patients. Cytotoxics remain the main class of anticancer drugs used in treating PDAC patients, and recent studies show that the microbiota can significantly alter their efficacy. In vitro and in vivo studies evaluating gemcitabine in multiple cancer cell lines showed that gemcitabine efficacy was attenuated in the presence of Escherichia coli [75]. A separate study by Geller et al. showed that PDAC tumor samples harbored the bacteria class Gammaproteobacteria, which is capable of metabolizing gemcitabine to the inactive 2′,2′-difluorodeoxyuridine by a long isoform of the enzyme cytidine deaminase (CDDL) [15]. This bacteria-induced gemcitabine resistance was abrogated by ciprofloxacin co-treatment in a colon cancer mouse model. Furthermore, bacteria derived from PDAC tumor samples induced gemcitabine resistance in 2 human colorectal cancer cell lines [15]. A retrospective study by Weniger et al. of PDAC patients receiving adjuvant gemcitabine found better PFS in PDAC patients without Klebsiella pneumoniae (which belongs to the class Gammaproteobacteria) in their bile culture than those with K. pneumoniae [73]. Antibiotic treatment with quinolones was associated with improved overall survival in patients who were positive for K. pneumoniae [73]. Overall, these results suggest that microbial dysbiosis can induce gemcitabine resistance, and appropriate antibiotic therapy may reverse the resistance and improve clinical outcomes. The successful identification of deleterious microbial profiles can potentially help triage PDAC patients for antibiotic therapy either prior to starting or during chemotherapy treatment.
Immune checkpoint inhibitors (ICIs) have so far been a failure in PDAC therapy despite successes in other cancer types [76]. The failure is partly attributed to the ‘immunosuppressive’ tumor microenvironment in PDAC [77,78,79]. As mentioned above, the microbiota may alter tumor microenvironment and immune cell infiltration in PDAC, which can potentially impact the efficacy of ICI therapy [19, 59, 60, 80, 81]. Research on the impact of microbiota on ICI in PDAC is sparse except for the study by Pushalkar et al., which showed that PD-1 was upregulated during antibiotic-mediated microbial ablation, and this was linked to improved antitumor effects of PD-1 blockade in a PDAC mouse model [19]. However, the microbial composition in melanoma patients was found to be different between immunotherapy responders and non-responders, and bacteria such as Bifidobacterium were associated with better response to ICIs [16, 82].
Not only can the microbiota impact drug efficacy, it can also predict and mediate chemotherapy-related toxicity [83]. For instance, the metabolism and clearance of irinotecan, a chemotherapeutic agent commonly used in PDAC that is known to induce dose-limiting diarrhea, is affected by gut microbiota [84]. Irinotecan is a prodrug of SN-38 and is converted to SN-38 by hepatic carboxylesterase; SN-38 is then metabolized by UGT1A1 in the liver to an inactive product, SN-38G [85, 86]. Patients with homozygous UGT1A1*28 allele, associated with decreased UGT1A1 activity, are at increased risk for irinotecan-related neutropenia due to decreased SN-38 clearance. The concentration of SN-38 is found unexpectedly high in feces as SN-38G can be converted back to SN-38 in the intestine by β-glucuronidase of naturally present, nonpathogenic gut bacteria such as E. coli, Bacteroides spp., and Clostridium perfringens, leading to the development of mucosal damage and severe diarrhea [86]. A preclinical study in rats by Stringer et al. showed that following irinotecan administration, there was an increase in the β-glucuronidase producing gut microbiota, which may be the primary cause of excessive gastrointestinal toxicity experienced by some patients [87]. Another study by Bhatt et al. demonstrated that inhibition of β-glucuronidase significantly decreased the incidence of diarrhea when co-administered with irinotecan in a mouse model [88]. A pilot study in cancer patients receiving irinotecan-containing regimens is currently underway at the Mayo Clinic that is designed to assess the activity of β-glucuronidase in stool samples with a specialized probe [89] and evaluate the impact of gut microbiota on the efficacy and toxicity of irinotecan. The results of these studies will provide rationale for further development of the fecal microbiota as a predictive biomarker for treatment-related toxicities in gastrointestinal cancer (including PDAC) patients receiving irinotecan.
4 Current clinical trials
There is nascent but growing interest in leveraging the microbiome for PDAC detection and treatment. Several studies are underway to comprehensively evaluate the impact of dysbiosis in pancreas and other gastrointestinal malignancies. These range from understanding the impact of the microbiota on carcinogenesis and tumor microenvironment to developing microbiome signatures for diagnosis and predicting prognosis and treatment response (Table 1). There are also interventional trials to evaluate how to leverage our understanding of the microbiome to develop novel therapies to improve patient outcomes (Table 2).
MRx0518 is a live biotherapeutic consisting of a lyophilized formulation of a proprietary bacterium and is currently undergoing clinical investigation in several phase I trials. The preliminary results from two of these trials were presented at the Society for Immunotherapy of Cancer (SITC) Annual Meeting in 2020. In one of the trials, MRx0518 was used as monotherapy in the neoadjuvant setting for multiple solid tumors (NCT03934827). Results from part A of the study showed safety and tolerability of MRx0518, and exploratory analyses of post-treatment samples demonstrated increased infiltration of immune cells [90]. In another trial, MRx0518 was used in combination with pembrolizumab in patients with advanced solid tumors refractory to ICIs (NCT03637803). Part A of the study enrolled patients with metastatic non-small cell lung cancer and renal cell carcinoma. The study demonstrated safety and tolerability of the combination, and 5 of 12 (42%) patients achieved clinical benefit [91]. There is now an active phase I trial in pancreatic cancer of using MRx0518 with hypofractionated radiation therapy (NCT04193904).
Several other interventional trials are ongoing, but no results have been reported yet. MS-20 is a biotherapeutic agent consisting of a mixture of soybean fermentation metabolites and microorganisms that mimic the human intestinal environment. There is an ongoing trial to examine the effects of MS-20 on gut microbiota and risk/severity of cachexia in pancreatic cancer patients undergoing chemotherapy treatment (NCT04600154). Another phase II trial is evaluating the hypothesis that oral vancomycin can impact gut commensal bacteria and increase cytokine expression, which results in liver-selective NKT-mediated antissssstumor effects, by assessing the combination of nivolumab, tadalafil, and oral vancomycin in patients with refractory hepatocellular carcinoma or liver dominant metastatic colorectal or pancreatic cancers (NCT03785210) [92].
While not many interventional trials involving the microbiome exist for pancreatic cancer, various microbiome-based strategies are under clinical investigation for other malignancies and may provide future study directions for pancreatic cancer. The most well-studied intervention involves fecal microbiota transplantation (FMT), where fecal materials from donors are transferred to recipients via endoscopy, colonoscopy, or oral capsules. Several phase I trials have demonstrated that FMT can improve response to ICI in patients with ICI-resistant metastatic melanoma and induce favorable changes in the tumor microenvironment [93, 94]. Similar studies that combine FMT and immunotherapy are underway in gastrointestinal cancers, prostate cancer, non-small cell lung cancer, and mesothelioma (NCT04130763, NCT04729322, NCT04521075, NCT04116775, NCT04056026). FMT is also being evaluated for its impact on ICI toxicities in various malignancies (NCT03819296, NCT04163289). In addition to FMT, administration of probiotics in combination with immunotherapy is under evaluation in renal cell carcinoma, breast cancer, non-small cell lung cancer, and colorectal cancer (NCT03829111, NCT03775850, NCT04909034). There is growing evidence that specific dietary changes can alter the intestinal microbiota [95], which can be associated with altered response to immunotherapy. Despite these studies, immune checkpoint inhibitor therapies in the current forms have yet to demonstrate activity in pancreatic cancer and will likely limit the applicability of FMT to enhance ICI in this disease.
Several trials are underway to evaluate the clinical outcomes of melanoma patients receiving high fiber diets and immunotherapy (NCT04645680, NCT04866810). Finally, as mentioned previously, antibiotic use may alter the gut microbiome and impact sensitivity to chemotherapy. There is an active phase II trial exploring the impact of metronidazole on the efficacy of adjuvant chemotherapy in patients with colorectal cancer (NCT04264676).
5 Conclusions
Despite decades of research, pancreatic cancer remains a deadly disease with limited treatment options and poor patient outcomes. Our growing understanding of the role of microbiome in pancreatic cancer will provide new insights and potentially lead to opportunities for the development of novel biomarkers and interventional strategies. Current studies have revealed challenges in the evaluation of microbiome in pancreatic cancer such as conflicting results on the specific microbial signatures associated with tumor development, progression, and treatment response. We need to improve the quality of future studies by controlling for patient comorbidities and standardizing the study design, methods of sampling, and primers used for sequencing and analysis. There is also a paucity of data regarding the role of other microorganisms such as viruses and protozoa, and better techniques are needed to advance our understanding in this area. Microbiome-based interventional studies in pancreatic cancer are an emerging field and are currently limited to evaluating the impact of a few biotherapeutic agents on patient response to radiation therapy and treatment toxicities. However, the modulation of microbiota has the potential to augment drug efficacy and reduce toxicity, and future studies should integrate microbiome-based biomarkers as well as evaluate the role of FMT, probiotics, dietary changes, and antibiotics in altering treatment response and patient outcomes. Well-designed, geographically diverse, prospective clinical trials will be needed to validate the results. Overall, the study of microbiome in pancreatic cancer holds great promise as a new frontier for precision medicine in the management of pancreatic cancer and deserves further investigation.
References
Siegel, R. L., Miller, K. D., Fuchs, H. E., & Jemal, A. (2021). Cancer Statistics, 2021. CA: A Cancer Journal for Clinicians, 71(1), 7–33. https://doi.org/10.3322/caac.21654
Li, D., Xie, K., Wolff, R., & Abbruzzese, J. L. (2004). Pancreatic cancer. The Lancet, 363(9414), 1049–1057. https://doi.org/10.1016/S0140-6736(04)15841-8
Cancer Statistics Review, 1975-2016 - SEER Statistics. (n.d.). Retrieved January 28, 2021, from https://seer.cancer.gov/archive/csr/1975_2016/
Costello, E. K., Lauber, C. L., Hamady, M., Fierer, N., Gordon, J. I., & Knight, R. (2009). Bacterial community variation in human body habitats across space and time. Science (New York, N.Y.), 326(5960), 1694–1697. https://doi.org/10.1126/science.1177486
Turnbaugh, P. J., Ley, R. E., Hamady, M., Fraser-Liggett, C. M., Knight, R., & Gordon, J. I. (2007). The Human Microbiome Project. Nature, 449(7164), 804–810. https://doi.org/10.1038/nature06244
Sender, R., Fuchs, S., & Milo, R. (2016). Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell, 164(3), 337–340. https://doi.org/10.1016/j.cell.2016.01.013
Liang, D., Leung, R. K.-K., Guan, W., & Au, W. W. (2018). Involvement of gut microbiome in human health and disease: Brief overview, knowledge gaps and research opportunities. Gut Pathogens, 10(1), 3. https://doi.org/10.1186/s13099-018-0230-4
Helmink, B. A., Khan, M. A. W., Hermann, A., Gopalakrishnan, V., & Wargo, J. A. (2019). The microbiome, cancer, and cancer therapy. Nature Medicine, 25(3), 377–388. https://doi.org/10.1038/s41591-019-0377-7
De Martel, C., Ferlay, J., Franceschi, S., Vignat, J., Bray, F., Forman, D., & Plummer, M. (2012). Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncology, 13, 607–615. https://doi.org/10.1016/S1470
McAllister, F., Khan, M. A. W., Helmink, B., & Wargo, J. A. (2019). The tumor microbiome in pancreatic cancer: Bacteria and beyond. Cancer Cell, 36(6), 577–579. https://doi.org/10.1016/j.ccell.2019.11.004
Maekawa, T., Fukaya, R., Takamatsu, S., Itoyama, S., Fukuoka, T., Yamada, M., Hata, T., Nagaoka, S., Kawamoto, K., Eguchi, H., Murata, K., Kumada, T., Ito, T., Tanemura, M., Fujimoto, K., Tomita, Y., Tobe, T., Kamada, Y., & Miyoshi, E. (2018). Possible involvement of Enterococcus infection in the pathogenesis of chronic pancreatitis and cancer. Biochemical and Biophysical Research Communications, 506(4), 962–969. https://doi.org/10.1016/j.bbrc.2018.10.169
Yu, A. I., Zhao, L., Eaton, K. A., Ho, S., Chen, J., Poe, S., Becker, J., Gonzalez, A., McKinstry, D., Hasso, M., Mendoza-Castrejon, J., Whitfield, J., Koumpouras, C., Schloss, P. D., Martens, E. C., & Chen, G. Y. (2020). Gut microbiota modulate CD8 T cell responses to influence colitis-associated tumorigenesis. Cell reports, 31(1), 107471. https://doi.org/10.1016/j.celrep.2020.03.035
Viaud, S., Saccheri, F., Mignot, G., Yamazaki, T., Daillère, R., Hannani, D., et al. (2013). The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science, 342(6161), 971 LP–971976. https://doi.org/10.1126/science.1240537
Ma, C., Han, M., Heinrich, B., Fu, Q., Zhang, Q., Sandhu, M., Agdashian, D., Terabe, M., Berzofsky, J. A., Fako, V., Ritz, T., Longerich, T., Theriot, C. M., McCulloch, J. A., Roy, S., Yuan, W., Thovarai, V., Sen, S. K., Ruchirawat, M., et al. (2018). Gut microbiome–mediated bile acid metabolism regulates liver cancer via NKT cells. Science, 360(6391), eaan5931. https://doi.org/10.1126/science.aan5931
Geller, L. T., Barzily-Rokni, M., Danino, T., Jonas, O. H., Shental, N., Nejman, D., Gavert, N., Zwang, Y., Cooper, Z. A., Shee, K., Thaiss, C. A., Reuben, A., Livny, J., Avraham, R., Frederick, D. T., Ligorio, M., Chatman, K., Johnston, S. E., Mosher, C. M., et al. (2017). Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science, 357(6356), 1156–1160. https://doi.org/10.1126/SCIENCE.AAH5043
Nejman, D., Livyatan, I., Fuks, G., Gavert, N., Zwang, Y., Geller, L. T., Rotter-Maskowitz, A., Weiser, R., Mallel, G., Gigi, E., Meltser, A., Douglas, G. M., Kamer, I., Gopalakrishnan, V., Dadosh, T., Levin-Zaidman, S., Avnet, S., Atlan, T., Cooper, Z. A., et al. (2020). The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Science, 368(6494), 973 LP–973980. https://doi.org/10.1126/science.aay9189
Kuczynski, J., Lauber, C. L., Walters, W. A., Parfrey, L. W., Clemente, J. C., Gevers, D., & Knight, R. (2012). Experimental and analytical tools for studying the human microbiome. Nature Reviews Genetics, 13(1), 47–58. https://doi.org/10.1038/nrg3129
Dollive, S., Peterfreund, G. L., Sherrill-Mix, S., Bittinger, K., Sinha, R., Hoffmann, C., Nabel, C. S., Hill, D. A., Artis, D., Bachman, M. A., Custers-Allen, R., Grunberg, S., Wu, G. D., Lewis, J. D., & Bushman, F. D. (2012). A tool kit for quantifying eukaryotic rRNA gene sequences from human microbiome samples. Genome Biology, 13(7), R60. https://doi.org/10.1186/gb-2012-13-7-r60
Pushalkar, S., Hundeyin, M., Daley, D., Zambirinis, C. P., Kurz, E., Mishra, A., Mohan, N., Aykut, B., Usyk, M., Torres, L. E., Werba, G., Zhang, K., Guo, Y., Li, Q., Akkad, N., Lall, S., Wadowski, B., Gutierrez, J., Kochen Rossi, J. A., et al. (2018). The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discovery, 403, 403–416. https://doi.org/10.1158/2159-8290.CD-17-1134
Jeong, J.-Y., Kim, T.-B., Kim, J., Choi, H. W., Kim, E. J., Yoo, H. J., Lee, S., Jun, H. R., Yoo, W., Kim, S., Kim, S. C., & Jun, E. (2020). Diversity in the extracellular vesicle-derived microbiome of tissues according to tumor progression in pancreatic cancer. Cancers, 12. https://doi.org/10.3390/cancers12092346
Rogers, M. B., Aveson, V., Firek, B., Yeh, A., Brooks, B., Brower-Sinning, R., Steve, J., Banfield, J. F., Zureikat, A., Hogg, M., Boone, B. A., Zeh, H. J., & Morowitz, M. J. (2017). Disturbances of the perioperative microbiome across multiple body sites in patients undergoing pancreaticoduodenectomy. Pancreas, 46(2) Retrieved from https://journals.lww.com/pancreasjournal/Fulltext/2017/02000/Disturbances_of_the_Perioperative_Microbiome.19.aspx, 260–267.
Aykut, B., Pushalkar, S., Chen, R., Li, Q., Abengozar, R., Kim, J. I., Shadaloey, S. A., Wu, D., Preiss, P., Verma, N., Guo, Y., Saxena, A., Vardhan, M., Diskin, B., Wang, W., Leinwand, J., Kurz, E., Kochen Rossi, J. A., Hundeyin, M., et al. (2019). The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature, 574(7777), 264–267. https://doi.org/10.1038/s41586-019-1608-2
Ren, Z., Jiang, J., Xie, H., Li, A., Lu, H., Xu, S., … Zheng, S. (2017). Gut microbial profile analysis by MiSeq sequencing of pancreatic carcinoma patients in China. Oncotarget, 8(56), 95176–95191. https://doi.org/10.18632/oncotarget.18820
Half, E., Keren, N., Reshef, L., Dorfman, T., Lachter, I., Kluger, Y., Reshef, N., Knobler, H., Maor, Y., Stein, A., Konikoff, F. M., & Gophna, U. (2019). Fecal microbiome signatures of pancreatic cancer patients. Scientific Reports, 9(1), 16801. https://doi.org/10.1038/s41598-019-53041-4
Mei, Q. X., Huang, C. L., Luo, S. Z., Zhang, X. M., Zeng, Y., & Lu, Y. Y. (2018). Characterization of the duodenal bacterial microbiota in patients with pancreatic head cancer vs. healthy controls. Pancreatology, 18(4), 438–445. https://doi.org/10.1016/j.pan.2018.03.005
Kohi, S., Macgregor-Das, A., Dbouk, M., Yoshida, T., Chuidian, M., Abe, T., Borges, M., Lennon, A. M., Shin, E. J., Canto, M. I., & Goggins, M. (2020). Alterations in the duodenal fluid microbiome of patients with pancreatic cancer. Clinical Gastroenterology and Hepatology. https://doi.org/10.1016/j.cgh.2020.11.006
Aldars-García, L., Chaparro, M., & Gisbert, J. P. (2021). Systematic review: The gut microbiome and its potential clinical application in inflammatory bowel disease. Microorganisms, 9. https://doi.org/10.3390/microorganisms9050977
Alamri, A. (2021). Diversity of microbial signatures in asthmatic airways. International journal of general medicine, 14, 1367–1378. https://doi.org/10.2147/IJGM.S304339
Hrncir, T., Hrncirova, L., Kverka, M., Hromadka, R., Machova, V., Trckova, E., Kostovcikova, K., Kralickova, P., Krejsek, J., & Tlaskalova-Hogenova, H. (2021). Gut Microbiota and NAFLD: Pathogenetic mechanisms, microbiota signatures, and therapeutic interventions. Microorganisms, 9(5), 957. https://doi.org/10.3390/microorganisms9050957
Lu, H., Ren, Z., Li, A., Li, J., Xu, S., Zhang, H., Jiang, J., Yang, J., Luo, Q., Zhou, K., Zheng, S., & Li, L. (2019). Tongue coating microbiome data distinguish patients with pancreatic head cancer from healthy controls. Journal of Oral Microbiology, 11(1), 1563409. https://doi.org/10.1080/20002297.2018.1563409
Sun, H., Zhao, X., Zhou, Y., Wang, J., Ma, R., Ren, X., Wang, H., & Zou, L. (2020). Characterization of oral microbiome and exploration of potential biomarkers in patients with pancreatic cancer. BioMed Research International, 2020, 4712498–4712411. https://doi.org/10.1155/2020/4712498
Wei, A., Li, M., Li, G., Wang, X., Hu, W., Li, Z., et al. (2020). Oral microbiome and pancreatic cancer. World J Gastroenterol, 26(48), 7679–7692. https://doi.org/10.3748/wjg.v26.i48.7679]
Vogtmann, E., Han, Y., Caporaso, J. G., Bokulich, N., Mohamadkhani, A., Moayyedkazemi, A., Hua, X., Kamangar, F., Wan, Y., Suman, S., Zhu, B., Hutchinson, A., Dagnall, C., Jones, K., Hicks, B., Shi, J., Malekzadeh, R., Abnet, C. C., & Pourshams, A. (2020). Oral microbial community composition is associated with pancreatic cancer: A case-control study in Iran. Cancer Medicine, 9(2), 797–806. https://doi.org/10.1002/cam4.2660
Farrell, J. J., Zhang, L., Zhou, H., Chia, D., Elashoff, D., Akin, D., Paster, B. J., Joshipura, K., & Wong, D. T. W. (2012). Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut, 61(4), 582 LP–582588. https://doi.org/10.1136/gutjnl-2011-300784
Torres, P. J., Fletcher, E. M., Gibbons, S. M., Bouvet, M., Doran, K. S., & Kelley, S. T. (2015). Characterization of the salivary microbiome in patients with pancreatic cancer. PeerJ, 3, e1373–e1373. https://doi.org/10.7717/peerj.1373
Hruban, R. H., Petersen, G. M., Ha, P. K., & Kern, S. E. (1998). Genetics of pancreatic cancer: From genes to families. Surgical Oncology Clinics of North America, 7(1), 1–23. https://doi.org/10.1016/S1055-3207(18)30282-5
Genkinger, J. M., Spiegelman, D., Anderson, K. E., Bernstein, L., van den Brandt, P. A., Calle, E. E., English, D. R., Folsom, A. R., Freudenheim, J. L., Fuchs, C. S., Giles, G. G., Giovannucci, E., Horn-Ross, P. L., Larsson, S. C., Leitzmann, M., Männistö, S., Marshall, J. R., Miller, A. B., Patel, A. V., et al. (2011). A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. International Journal of Cancer, 129(7), 1708–1717. https://doi.org/10.1002/ijc.25794
Aune, D., Greenwood, D. C., Chan, D. S. M., Vieira, R., Vieira, A. R., Navarro Rosenblatt, D. A., Cade, J. E., Burley, V. J., & Norat, T. (2012). Body mass index, abdominal fatness and pancreatic cancer risk: A systematic review and non-linear dose–response meta-analysis of prospective studies. Annals of Oncology, 23(4), 843–852. https://doi.org/10.1093/annonc/mdr398
Duell, E. J., Lucenteforte, E., Olson, S. H., Bracci, P. M., Li, D., Risch, H. A., Silverman, D. T., Ji, B. T., Gallinger, S., Holly, E. A., Fontham, E. H., Maisonneuve, P., Bueno-de-Mesquita, H. B., Ghadirian, P., Kurtz, R. C., Ludwig, E., Yu, H., Lowenfels, A. B., Seminara, D., et al. (2012). Pancreatitis and pancreatic cancer risk: A pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Annals of Oncology, 23(11), 2964–2970. https://doi.org/10.1093/annonc/mds140
Lynch, S. M., Vrieling, A., Lubin, J. H., Kraft, P., Mendelsohn, J. B., Hartge, P., Canzian, F., Steplowski, E., Arslan, A. A., Gross, M., Helzlsouer, K., Jacobs, E. J., LaCroix, A., Petersen, G., Zheng, W., Albanes, D., Amundadottir, L., Bingham, S. A., Boffetta, P., et al. (2009). Cigarette smoking and pancreatic cancer: A pooled analysis from the pancreatic cancer cohort consortium. American Journal of Epidemiology, 170(4), 403–413. https://doi.org/10.1093/aje/kwp134
Ben, Q., Xu, M., Ning, X., Liu, J., Hong, S., Huang, W., Zhang, H., & Li, Z. (2011). Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. European Journal of Cancer, 47(13), 1928–1937. https://doi.org/10.1016/j.ejca.2011.03.003
Rogers, C. J., Prabhu, K. S., & Vijay-Kumar, M. (2014). The microbiome and obesity—An established risk for certain types of cancer. The Cancer Journal, 20(3) Retrieved from https://journals.lww.com/journalppo/Fulltext/2014/05000/The_Microbiome_and_Obesity_An_Established_Risk_for.3.aspx, 176–180.
Djuric, Z. (2017). Obesity-associated cancer risk: The role of intestinal microbiota in the etiology of the host proinflammatory state. Translational research : the journal of laboratory and clinical medicine, 179, 155–167. https://doi.org/10.1016/j.trsl.2016.07.017
Million, M., Maraninchi, M., Henry, M., Armougom, F., Richet, H., Carrieri, P., Valero, R., Raccah, D., Vialettes, B., & Raoult, D. (2012). Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. International Journal of Obesity, 36(6), 817–825. https://doi.org/10.1038/ijo.2011.153
Qin, J., Li, Y., Cai, Z., Li, S., Zhu, J., Zhang, F., Liang, S., Zhang, W., Guan, Y., Shen, D., Peng, Y., Zhang, D., Jie, Z., Wu, W., Qin, Y., Xue, W., Li, J., Han, L., Lu, D., et al. (2012). A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature, 490(7418), 55–60. https://doi.org/10.1038/nature11450
Cani, P. D., & Jordan, B. F. (2018). Gut microbiota-mediated inflammation in obesity: A link with gastrointestinal cancer. Nature Reviews Gastroenterology & Hepatology, 15(11), 671–682. https://doi.org/10.1038/s41575-018-0025-6
Brubaker, L., Luu, S., Hoffman, K., Wood, A., Navarro Cagigas, M., Yao, Q., et al. (2021). Microbiome changes associated with acute and chronic pancreatitis: A systematic review. Pancreatology, 21(1), 1–14. https://doi.org/10.1016/j.pan.2020.12.013
Larsen, N., Vogensen, F. K., van den Berg, F. W. J., Nielsen, D. S., Andreasen, A. S., Pedersen, B. K., al-Soud, W. A., Sørensen, S. J., Hansen, L. H., & Jakobsen, M. (2010). Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLOS ONE, 5(2), e9085. Retrieved from. https://doi.org/10.1371/journal.pone.0009085
Hujoel, P. P., Drangsholt, M., Spiekerman, C., & Weiss, N. S. (2003). An exploration of the periodontitis–cancer association. Annals of Epidemiology, 13(5), 312–316. https://doi.org/10.1016/S1047-2797(02)00425-8
Michaud, D. S., Liu, Y., Meyer, M., Giovannucci, E., & Joshipura, K. (2008). Periodontal disease, tooth loss, and cancer risk in male health professionals: A prospective cohort study. The Lancet Oncology, 9(6), 550–558. https://doi.org/10.1016/S1470-2045(08)70106-2
Chang, J. S., Tsai, C.-R., Chen, L.-T., & Shan, Y.-S. (2016). Investigating the association between periodontal disease and risk of pancreatic cancer. Pancreas, 45(1) Retrieved from https://journals.lww.com/pancreasjournal/Fulltext/2016/01000/Investigating_the_Association_Between_Periodontal.19.aspx, 134–141.
Maisonneuve, P., Amar, S., & Lowenfels, A. B. (2017). Periodontal disease, edentulism, and pancreatic cancer: A meta-analysis. Annals of oncology : official journal of the European Society for Medical Oncology, 28(5), 985–995. https://doi.org/10.1093/annonc/mdx019
Hayashi, C., Gudino, C. V., Gibson III, F. C., & Genco, C. A. (2010). REVIEW: Pathogen-induced inflammation at sites distant from oral infection: Bacterial persistence and induction of cell-specific innate immune inflammatory pathways. Molecular Oral Microbiology, 25(5), 305–316. https://doi.org/10.1111/j.2041-1014.2010.00582.x
Coussens, L. M., & Werb, Z. (2002). Inflammation and cancer. Nature, 420(6917), 860–867. https://doi.org/10.1038/nature01322
Michaud, D. S., Izard, J., Wilhelm-Benartzi, C. S., You, D.-H., Grote, V. A., Tjønneland, A., … Riboli, E. (2013). Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut, 62(12), 1764 LP – 1770. https://doi.org/10.1136/gutjnl-2012-303006
Fan, X., Alekseyenko, A. V., Wu, J., Peters, B. A., Jacobs, E. J., Gapstur, S. M., et al. (2018). Human oral microbiome and prospective risk for pancreatic cancer: A population-based nested case-control study. Gut, 67(1), 120 LP–120127. https://doi.org/10.1136/gutjnl-2016-312580
Gnanasekaran, J., Binder Gallimidi, A., Saba, E., Pandi, K., Eli Berchoer, L., Hermano, E., Angabo, S., Makkawi, H.′., Khashan, A., Daoud, A., Elkin, M., & Nussbaum, G. (2020). Intracellular Porphyromonas gingivalis promotes the tumorigenic behavior of pancreatic carcinoma cells. Cancers, 12. https://doi.org/10.3390/cancers12082331
Binder Gallimidi, A., Fischman, S., Revach, B., Bulvik, R., Maliutina, A., Rubinstein, A. M., … Elkin, M. (2015). Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget, 6(26), 22613–22623. https://doi.org/10.18632/oncotarget.4209
Thomas, R. M., Gharaibeh, R. Z., Gauthier, J., Beveridge, M., Pope, J. L., Guijarro, M. V., Guijarro, M. V., Yu, Q., He, Z., Ohland, C., Newsome, R., Trevino, J., Hughes, S. J., Reinhard, M., Winglee, K., Fodor, A. A., Zajac-Kaye, M., & Jobin, C. (2018). Intestinal microbiota enhances pancreatic carcinogenesis in preclinical models. Carcinogenesis, 39(8), 1068–1078. https://doi.org/10.1093/carcin/bgy073
Sethi, V., Kurtom, S., Tarique, M., Lavania, S., Malchiodi, Z., Hellmund, L., Zhang, L., Sharma, U., Giri, B., Garg, B., Ferrantella, A., Vickers, S. M., Banerjee, S., Dawra, R., Roy, S., Ramakrishnan, S., Saluja, A., & Dudeja, V. (2018). Gut microbiota promotes tumor growth in mice by modulating immune response. Gastroenterology, 155(1), 33–37. https://doi.org/10.1053/j.gastro.2018.04.001
Petrelli, F., Ghidini, M., Ghidini, A., Perego, G., Cabiddu, M., Khakoo, S., Oggionni, E., Abeni, C., Hahne, J. C., Tomasello, G., & Zaniboni, A. (2019). Use of antibiotics and risk of cancer: A systematic review and meta-analysis of observational studies. Cancers, 11. https://doi.org/10.3390/cancers11081174
Pinato, D. J., Howlett, S., Ottaviani, D., Urus, H., Patel, A., Mineo, T., Brock, C., Power, D., Hatcher, O., Falconer, A., Ingle, M., Brown, A., Gujral, D., Partridge, S., Sarwar, N., Gonzalez, M., Bendle, M., Lewanski, C., Newsom-Davis, T., et al. (2019). Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncology, 5(12), 1774–1778. https://doi.org/10.1001/jamaoncol.2019.2785
Hakozaki, T., Richard, C., Elkrief, A., Hosomi, Y., Benlaïfaoui, M., Mimpen, I., Terrisse, S., Derosa, L., Zitvogel, L., Routy, B., & Okuma, Y. (2020). The gut microbiome associates with immune checkpoint inhibition outcomes in patients with advanced non–small cell lung cancer. Cancer Immunology Research, 8(10), 1243 LP–1241250. https://doi.org/10.1158/2326-6066.CIR-20-0196
Jin, Y., Dong, H., Xia, L., Yang, Y., Zhu, Y., Shen, Y., Zheng, H., Yao, C., Wang, Y., & Lu, S. (2019). the diversity of gut microbiome is associated with favorable responses to anti–programmed death 1 immunotherapy in Chinese patients with NSCLC. Journal of Thoracic Oncology, 14(8), 1378–1389. https://doi.org/10.1016/j.jtho.2019.04.007
KIM, J.-E., LEE, K. Y. U. T., LEE, J. K., PAIK, S. W., RHEE, J. C., & CHOI, K. W. A. N. (2004). Clinical usefulness of carbohydrate antigen 19-9 as a screening test for pancreatic cancer in an asymptomatic population. Journal of Gastroenterology and Hepatology, 19(2), 182–186. https://doi.org/10.1111/j.1440-1746.2004.03219.x
Mendez, R., Kesh, K., Arora, N., Di Martino, L., McAllister, F., Merchant, N., … Banerjee, S. (2020). Microbial dysbiosis and polyamine metabolism as predictive markers for early detection of pancreatic cancer. Carcinogenesis, 41(5), 561–570. https://doi.org/10.1093/carcin/bgz116
De Filippo, C., Cavalieri, D., Di Paola, M., Ramazzotti, M., Poullet, J. B., Massart, S., … Lionetti, P. (2010). Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proceedings of the National Academy of Sciences, 107(33), 14691 LP – 14696. https://doi.org/10.1073/pnas.1005963107.
Yatsunenko, T., Rey, F. E., Manary, M. J., Trehan, I., Dominguez-Bello, M. G., Contreras, M., Magris, M., Hidalgo, G., Baldassano, R. N., Anokhin, A. P., Heath, A. C., Warner, B., Reeder, J., Kuczynski, J., Caporaso, J. G., Lozupone, C. A., Lauber, C., Clemente, J. C., Knights, D., et al. (2012). Human gut microbiome viewed across age and geography. Nature, 486(7402), 222–227. https://doi.org/10.1038/nature11053
Obregon-Tito, A. J., Tito, R. Y., Metcalf, J., Sankaranarayanan, K., Clemente, J. C., Ursell, L. K., Zech Xu, Z., van Treuren, W., Knight, R., Gaffney, P. M., Spicer, P., Lawson, P., Marin-Reyes, L., Trujillo-Villarroel, O., Foster, M., Guija-Poma, E., Troncoso-Corzo, L., Warinner, C., Ozga, A. T., & Lewis, C. M. (2015). Subsistence strategies in traditional societies distinguish gut microbiomes. Nature Communications, 6(1), 6505. https://doi.org/10.1038/ncomms7505
Grzeskowiak, L., Collado, M. C., Mangani, C., Maleta, K., Laitinen, K., Ashorn, P., … Salminen, S. (2012). Distinct gut microbiota in Southeastern African and Northern European infants. Journal of Pediatric Gastroenterology and Nutrition, 54(6). Retrieved from https://journals.lww.com/jpgn/Fulltext/2012/06000/Distinct_Gut_Microbiota_in_Southeastern_African.21.aspx.
Li, J., Quinque, D., Horz, H.-P., Li, M., Rzhetskaya, M., Raff, J. A., Hayes, M. G., & Stoneking, M. (2014). Comparative analysis of the human saliva microbiome from different climate zones: Alaska, Germany, and Africa. BMC Microbiology, 14(1), 316. https://doi.org/10.1186/s12866-014-0316-1
Mitsuhashi, K., Nosho, K., Sukawa, Y., Matsunaga, Y., Ito, M., Kurihara, H., Kanno, S., Igarashi, H., Naito, T., Adachi, Y., Tachibana, M., Tanuma, T., Maguchi, H., Shinohara, T., Hasegawa, T., Imamura, M., Kimura, Y., Hirata, K., Maruyama, R., et al. (2015). Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget, 6(9) Retrieved from https://www.oncotarget.com/article/3109/text/, 7209–7220.
Weniger, M., Hank, T., Qadan, M., Ciprani, D., Michelakos, T., Niess, H., et al. (2020). Influence of Klebsiella pneumoniae and quinolone treatment on prognosis in patients with pancreatic cancer. British Journal of Surgery. https://doi.org/10.1002/bjs.12003
Riquelme, E., Zhang, Y., Zhang, L., Montiel, M., Zoltan, M., Dong, W., Quesada, P., Sahin, I., Chandra, V., San Lucas, A., Scheet, P., Xu, H., Hanash, S. M., Feng, L., Burks, J. K., Do, K. A., Peterson, C. B., Nejman, D., Tzeng, C. W. D., et al. (2019). Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell, 178(4), 795–806.e12. https://doi.org/10.1016/j.cell.2019.07.008
Lehouritis, P., Cummins, J., Stanton, M., Murphy, C. T., McCarthy, F. O., Reid, G., Urbaniak, C., Byrne, W. L., & Tangney, M. (2015). Local bacteria affect the efficacy of chemotherapeutic drugs. Scientific reports, 5, 14554. https://doi.org/10.1038/srep14554
Balachandran, V. P., Beatty, G. L., & Dougan, S. K. (2019). Broadening the impact of immunotherapy to pancreatic cancer: challenges and opportunities. Gastroenterology, 156(7), 2056–2072. https://doi.org/10.1053/j.gastro.2018.12.038
Upadhrasta, S., & Zheng, L. (2019). Strategies in developing immunotherapy for pancreatic cancer: Recognizing and correcting multiple immune “defects” in the tumor microenvironment. Journal of Clinical Medicine, 8. https://doi.org/10.3390/jcm8091472
Feig, C., Jones, J. O., Kraman, M., Wells, R. J. B., Deonarine, A., Chan, D. S., Connell, C. M., Roberts, E. W., Zhao, Q., Caballero, O. L., Teichmann, S. A., Janowitz, T., Jodrell, D. I., Tuveson, D. A., & Fearon, D. T. (2013). Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti–PD-L1 immunotherapy in pancreatic cancer. Proceedings of the National Academy of Sciences, 110(50), 20212 LP–20220217. https://doi.org/10.1073/pnas.1320318110
Li, J., Byrne, K. T., Yan, F., Yamazoe, T., Chen, Z., Baslan, T., Richman, L. P., Lin, J. H., Sun, Y. H., Rech, A. J., Balli, D., Hay, C. A., Sela, Y., Merrell, A. J., Liudahl, S. M., Gordon, N., Norgard, R. J., Yuan, S., Yu, S., et al. (2018). Tumor cell-intrinsic factors underlie heterogeneity of immune cell infiltration and response to immunotherapy. Immunity, 49(1), 178–193.e7. https://doi.org/10.1016/j.immuni.2018.06.006
Matson, V., Fessler, J., Bao, R., Chongsuwat, T., Zha, Y., Alegre, M. L., Luke, J. J., & Gajewski, T. F. (2018). The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science, 359(6371), 104–108. https://doi.org/10.1126/science.aao3290
Routy, B., Le Chatelier, E., Derosa, L., Duong, C. P. M., Alou, M. T., Daillère, R., et al. (2018). Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science, 359(6371), 91–97. https://doi.org/10.1126/science.aan3706
Sivan, A., Corrales, L., Hubert, N., Williams, J. B., Aquino-Michaels, K., Earley, Z. M., Benyamin, F. W., Man Lei, Y., Jabri, B., Alegre, M. L., Chang, E. B., & Gajewski, T. F. (2015). Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Sciences, 350(6264), 1084 LP–1081089. https://doi.org/10.1126/science.aac4255
Alexander, J. L., Wilson, I. D., Teare, J., Marchesi, J. R., Nicholson, J. K., & Kinross, J. M. (2017). Gut microbiota modulation of chemotherapy efficacy and toxicity. Nature Reviews Gastroenterology & Hepatology, 14(6), 356–365. https://doi.org/10.1038/nrgastro.2017.20
Takasuna, K., Hagiwara, T., Hirohashi, M., Kato, M., Nomura, M., Nagai, E., … Kamataki, T. (1996). Involvement of β-Glucuronidase in Intestinal Microflora in the Intestinal Toxicity of the Antitumor Camptothecin Derivative Irinotecan Hydrochloride (CPT-11) in Rats. Cancer Research, 56(16), 3752 LP – 3757. Retrieved from http://cancerres.aacrjournals.org/content/56/16/3752.abstract
Humerickhouse, R., Lohrbach, K., Li, L., Bosron, W. F., & Dolan, M. E. (2000). Characterization of CPT-11 hydrolysis by human liver carboxylesterase isoforms hCE-1 and hCE-2. Cancer Research, 60(5), 1189 LP–1181192 Retrieved from http://cancerres.aacrjournals.org/content/60/5/1189.abstract
Mathijssen, R. H. J., van Alphen, R. J., Verweij, J., Loos, W. J., Nooter, K., Stoter, G., & Sparreboom, A. (2001). Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clinical Cancer Research, 7(8), 2182 LP–2182194 Retrieved from http://clincancerres.aacrjournals.org/content/7/8/2182.abstract
Stringer, A. M., Gibson, R. J., Logan, R. M., Bowen, J. M., Yeoh, A. S. J., & Keefe, D. M. K. (2008). Faecal microflora and β-glucuronidase expression are altered in an irinotecan-induced diarrhea model in rats. Cancer Biology & Therapy, 7(12), 1919–1925. https://doi.org/10.4161/cbt.7.12.6940
Bhatt, A. P., Pellock, S. J., Biernat, K. A., Walton, W. G., Wallace, B. D., Creekmore, B. C., Letertre, M. M., Swann, J. R., Wilson, I. D., Roques, J. R., Darr, D. B., Bailey, S. T., Montgomery, S. A., Roach, J. M., Azcarate-Peril, M. A., Sartor, R. B., Gharaibeh, R. Z., Bultman, S. J., & Redinbo, M. R. (2020). Targeted inhibition of gut bacterial β-glucuronidase activity enhances anticancer drug efficacy. Proceedings of the National Academy of Sciences, 117(13), 7374 LP–7377381. https://doi.org/10.1073/pnas.1918095117
Whidbey, C., Sadler, N. C., Nair, R. N., Volk, R. F., DeLeon, A. J., Bramer, L. M., Fansler, S. J., Hansen, J. R., Shukla, A. K., Jansson, J. K., Thrall, B. D., & Wright, A. T. (2019). A probe-enabled approach for the selective isolation and characterization of functionally active subpopulations in the gut microbiome. Journal of the American Chemical Society, 141(1), 42–47. https://doi.org/10.1021/jacs.8b09668
Lythgoe, M., Stebbing, J., Pickford, E., Glasmacher, A., Adriani, M., Fyvie, G., Frampton, A., Stevenson, A., & Krell, J. (2020). 805 Safety and emerging evidence of immune modulation of the live biotherapeutic MRx0518 in the neoadjuvant setting for patients awaiting surgical removal of solid tumours. Journal for ImmunoTherapy of Cancer, 8(Suppl 3), A481 LP–A48A482. https://doi.org/10.1136/jitc-2020-SITC2020.0805
Pant, S., Shah, A., Msaouel, P., Campbell, M., Tu, S.-M., Gao, J., Blumenschein, G., Mott, F., le, X., Altan, M., Meric-Bernstam, F., Yap, T., Subbiah, V., Rodon, J., Glasmacher, A., Mulder, I., Chisamore, M., Stevenson, A., & Tannir, N. (2020). 283 Safety and efficacy signals in the complete phase I study of live biotherapeutic MRx0518 in combination with pembrolizumab in patients refractory to immune checkpoint inhibitors (ICIs). Journal for ImmunoTherapy of Cancer, 8(Suppl 3), A173 LP–A17A173. https://doi.org/10.1136/jitc-2020-SITC2020.0283
Monge, B. M. C., Xie, C., Mabry-Hrones, D., Wood, B. J., Steinberg, S. M., Kleiner, D. E., & Greten, T. F. (2020). Phase II study of nivolumab (anti-PD1), tadalafil, and oral vancomycin in patients with refractory primary hepatocellular carcinoma or liver dominant metastatic cancer from colorectal or pancreatic cancers. Journal of Clinical Oncology, 38(15_suppl), TPS4656–TPS4656. https://doi.org/10.1200/JCO.2020.38.15_suppl.TPS4656
Baruch, E. N., Youngster, I., Ben-Betzalel, G., Ortenberg, R., Lahat, A., Katz, L., Adler, K., Dick-Necula, D., Raskin, S., Bloch, N., Rotin, D., Anafi, L., Avivi, C., Melnichenko, J., Steinberg-Silman, Y., Mamtani, R., Harati, H., Asher, N., Shapira-Frommer, R., et al. (2021). Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science, 371(6529), 602 LP–602609. https://doi.org/10.1126/science.abb5920
Davar, D., Dzutsev, A. K., McCulloch, J. A., Rodrigues, R. R., Chauvin, J.-M., Morrison, R. M., Deblasio, R. N., Menna, C., Ding, Q., Pagliano, O., Zidi, B., Zhang, S., Badger, J. H., Vetizou, M., Cole, A. M., Fernandes, M. R., Prescott, S., Costa, R. G. F., Balaji, A. K., et al. (2021). Fecal microbiota transplant overcomes resistance to anti–PD-1 therapy in melanoma patients. Science, 371(6529), 595 LP–595602. https://doi.org/10.1126/science.abf3363
O’Keefe, S. J. D., Li, J. V, Lahti, L., Ou, J., Carbonero, F., Mohammed, K., … Zoetendal, E. G. (2015). Fat, fibre and cancer risk in African Americans and rural Africans. Nature Communications, 6(1), 6342. https://doi.org/10.1038/ncomms7342
Data and materials availability
Not applicable
Code availability
Not applicable
Funding
NC acknowledges National Cancer Institute grant no. R01CA179243.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, J.J., Zhu, M., Kashyap, P.C. et al. The role of microbiome in pancreatic cancer. Cancer Metastasis Rev 40, 777–789 (2021). https://doi.org/10.1007/s10555-021-09982-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-021-09982-2