Abstract
The purpose of this study was to investigate left ventricular contraction patterns in asymptomatic Childhood cancer survivors (CCS) using two-dimensional speckle tracking echocardiography (2DSTE). Left ventricular longitudinal and circumferential myocardial parameters were assessed using 2DSTE, in asymptomatic CCS and age matched healthy controls. Time to peak (T2P) systolic strain was quantified. Dyssynchrony index (DI) was measured by calculating the standard deviation of T2P systolic strain of six segments in each view. Difference between T2P systolic longitudinal strain of septal and lateral wall was also assessed as a parameter for dyssynchrony. We included 115 CCS with a median age of 17.2 years (range 5.6–39.5) and a median follow up of 11.3 years (range 4.9–29.5) and 119 controls. Conventional echocardiographic parameters and global longitudinal strain were significantly decreased in CCS compared to controls (p < 0.01 and p = 0.02, respectively). Dyssynchrony index did not differ between CCS and controls. There was a clinically insignificant smaller absolute difference between T2P systolic longitudinal of septal and lateral wall in CCS compared to controls. We showed no difference in longitudinal or circumferential left ventricular dyssynchrony in CCS compared to controls using 2DSTE. Future research should focus on assessing dyssynchrony in more segments and a larger CCS population, using both 2D and 3DSTE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long-term survival of children with cancer has improved over the last decades to approximately 70–80%. Anthracyclines are used in many cancer treatment protocols and have a known cardiotoxic effect. Cardiac disease is seen in 10.6% of Childhood cancer survivors (CCS) and increases to 27.8% if radiotherapy has been given [1]. Mechanisms of anthracycline-induced cardiotoxicity have been studied extensively. There is a role for cardiomyocyte apoptosis due to DNA damage by topoisomerase 2b and formation of reactive oxygen species [2]. However, cardiac extracellular matrix remodeling (cardiac fibrosis) may represent an additional important mechanism contributing to impaired Left ventricular (LV) function and adverse cardiac outcome [3,4,5]. This fibrosis is measurable by calculating extracellular volume fraction by cardiac magnetic resonance and has been found in CCS [6, 7]. Fibrosis has also been shown in myocardial biopsies from patients with anthracycline cardiomyopathy [8]. Fibrosis of the myocardial wall has been correlated to dyssynchronous myocardial contraction in patients after myocardial infarction and in patients with non-ischemic dilated cardiomyopathy [9, 10]. This may also occur in CCS. Currently, few studies assessed Left ventricular dyssynchrony (LVD) as a marker for anthracycline-induced cardiotoxicity. Strain-derived mechanical dyssynchrony abnormalities have been independently associated with Left ventricular ejection fraction (LVEF) changes over time in idiopathic dilated cardiomyopathy [11]. Decreased Global longitudinal strain (GLS) has already been used as an early sign of anthracycline-induced cardiotoxicity [12]. We hypothesize that increased left ventricular dyssynchrony could be an early sign of subclinical cardiotoxicity in asymptomatic CCS previously treated with anthracyclines. Differences in Time to peak (T2P) strain might be useful as a quick and easily accessible, bedside method to assess LVD. In this study, we aim to quantify T2P systolic longitudinal and circumferential strain and possible LVD in CCS using 2DSTE.
Methods
Study population
CCS who received anthracyclines as part of their cancer therapy, were included in this study when they visited our Late Effects outpatient clinic between December 2005 and November 2009. Exclusion criteria were: (1) Clinical heart failure, defined by the New York Heart Association (adults, NYHA, class II–IV), and the modified Ross heart failure classification (children), (2) history of cardiovascular disease or (3) chronic renal disease. The control group consisted of 119 healthy age-matched controls (children and adults), routinely referred for echocardiographic evaluation of an asymptomatic, innocent heart murmur or for screening purposes. Their medical history, electrocardiogram, and echocardiogram were not indicative of cardiac disease. For both groups, subjects were excluded when having poor echocardiographic image quality (framerate < 60/minute) or incomplete image acquisition. The study was approved by the local ethical committee and informed consent was obtained from all survivors (and their parents, when indicated).
Echocardiographic image acquisition and analysis
A detailed transthoracic echocardiographic examination in left lateral position was performed according to the recommendations of the American Society of Echocardiography [13]. Images were obtained with a 3.0 MHz or a 5.0 MHz phased-array transducer, depending on age and weight of the subject, using a commercially available Vivid 7 echocardiographic scanner (GE, Vingmed Ultrasound, Horton, Norway). Quantification of cardiac chamber size, ventricular mass and LV function were done in accordance with American and European guidelines [13]. Left ventricular End-systolic wall stress (ESWS) was calculated using the modified formula of Rowland and Gutgesell [14]. Since the study group consisted of children and adults, left ventricular dimensions were indexed for Body surface area (BSA), not by using Z scores. Systolic function was determined using Left ventricular shortening fraction (LVSF) and LVEF using Biplane Simpson’s method. Left ventricular mass (LVM) was calculated using the formula of Devereux and Reichek [15].
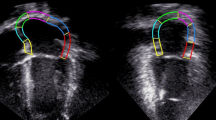
Two-dimensional multi-frame B-mode images were obtained in apical 4-chamber view (4CH) for the Longitudinal strain (LS) and in parasternal mid-cavity short-axis view (papillary muscle level: SaxPM) for the Circumferential strain (CS), as described earlier by our group [16]. GLS, global systolic LS rate (GLSr), global systolic CS (GCS) and global CS rate (GCSr) were calculated, using the mean of three heart cycles. Global T2P systolic strain was calculated by averaging T2P systolic strain values of all segments for both views.
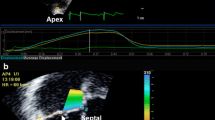
To measure the extent of mechanical LVD, Dyssynchrony indices (DI), the Standard deviation (SD) of the T2P systolic strain in the longitudinal and circumferential direction were calculated (Fig. 1). In addition, difference between T2P systolic LS between septal and lateral wall was calculated in 4CH view [17]. Correction for the influence of heart rate on timing parameters was achieved by representing values as a percentage of the heart cycle.
Measurement of dyssynchrony index in longitudinal strain. Calculation of dyssynchrony index (DI): Three cycles of longitudinal strain (LS) were measured. T2P systolic LS (indicated by the arrows) was calculated per segment (average of three cycles). DI was quantified by calculating the standard deviation of T2P systolic strain of six segments
Inter- and intra-observer reproducibility
Inter-observer reproducibility of strain measurement was performed by two experienced analysts (M.P and A.M-G) in 10 CCS. Intra-observer reproducibility was performed by one analyst (M.P) repeating the same analysis in 10 CCS, blinded to previous measurements.
Statistical analysis
Demographic and anthropometric values were summarized as median and range using a non-parametric test. Echocardiographic parameters were summarized as mean and SD. Characteristics of survivors and controls were compared using independent sample t-test. A two-tailed p-value of less than 0.05 was considered statistically significant. Correlations were studied using univariate analysis. Intraclass correlations (ICC) were calculated to assess intra- and interobserver variability using acknowledged cut-off points: < 0.20 poor agreement, 0.21–0.40 fair agreement, 0.41–0.60 a moderate agreement, 0.61–0.80 good agreement and 0.81–1.00 very good agreement. We considered an ICC > 0.60 as acceptable [18]. We used SPPS statistics, version 25 for statistical analysis.
Results
We included 115 CCS and 119 healthy age matched controls in our study. None of the survivors met the exclusion criteria. Demographic characteristics are shown in Table 1. Except for a significantly lower blood pressure and a higher heart rate in the CCS group, characteristics were similar for both groups.
Feasability of global T2P systolic LS and longitudinal DI was 99% in CCS and 97% in controls. Feasability of global T2P systolic CS and circumferential DI was 77% in CCS and 82% in controls. Feasability of difference in T2P systolic LS of septal and lateral wall was 96% in CCS and 94% in controls.
Table 2 presents conventional and 2DSTE parameters of both groups. Most conventional echocardiographic parameters were significantly decreased in CCS compared to controls. ESWS was significantly increased in CCS compared to controls. GLS was significantly decreased in CCS compared to controls.
Table 3 presents parameters of timing and LVD in survivors and controls. Global T2P systolic LS was shorter in CCS compared to controls. When corrected for heartrate this difference disappeared. Longitudinal DI (uncorrected for heartrate) was lower in CCS versus controls. This difference disappeared when corrected for heartrate. There was a significantly smaller absolute difference in global T2P systolic LS between septal and lateral wall in asymptomatic CCS compared to controls. Comparing children and adults separately did not alter the results. There was no correlation between the longitudinal or the circumferential DI and follow-up duration, cumulative anthracycline dose, gender or age at diagnosis (data not shown). None of the survivors or controls had an increased QRS interval indicative of left or right bundle branch block (data not shown).
ICCs for dyssynchrony parameters for intra-observer and inter-observer reproducibility ranged from 0.67 to 0.83 and 0.68 to 0.94 respectively.
Discussion
This pilot study assessed LV contracting patterns and the extent of LVD in CCS and healthy controls. Longitudinal DI, uncorrected for heart rate, was significantly lower in survivors compared to controls. We question the clinical relevance of this difference of 3 ms. When comparing our control values to a study by den Boer et al., the mean DI of the controls was similar (29 versus 30 ms). We found a broader range of DI in our control population compared to den Boer et al., possibly due to a larger age range in our population [20]. Klitsie et al. found an increasing DI with age in the pediatric population [19]. Our range of DI (children as well as adults) in survivors and controls fits their normal range of values supporting the conclusion that this small statistically significant difference in the longitudinal DI is clinically irrelevant. When longitudinal DI was corrected for heart rate, statistical significance disappeared. We found a smaller difference in global T2P systolic LS between septal and lateral wall in survivors compared to controls (22.3 versus 27.9 ms, p-value 0.019) which seems clinically irrelevant. Cut off values for LVD in predicting successful resynchronization therapy have been recently described by Mele et al. [21]. However, no cut-off values for LVD in predicting LV dysfunction in CCS have been identified.
To measure the DI in assessing LVD, different imaging techniques such as tissue Doppler (calculating the Yu-index), 2DSTE (as done in our study), 3D echocardiography or MRI can be used [20]. Dyssynchrony as a marker of early-onset anthracycline-induced cardiotoxicity has been demonstrated by Li et al. with an increased peak systolic dispersion measured by SD of T2P systolic LS of 18 segments (4CH, 3CH and 2CH) [22]. Dyssynchrony as a marker of late-onset anthracycline-induced cardiotoxicity has been shown by Okama et al. investigating 32 CCS and 12 controls. CCS were divided according to existence of diastolic LV regional Wall motion abnormalities (WMA). More dyssynchrony, defined as an increased LV Systolic dyssynchrony index (SDI) of the radial strain was seen in CCS with WMA, compared to controls and compared to CCS without WMA, with preserved LVEF. No difference in SDI was seen between controls and survivors without WMA [23]. A possible histopathological explanation for these findings is fibrosis leading to WMA as well as an increased SDI. Cheung et al. reported on 45 CCS using 3D echocardiography and measured SD of time to minimal volume of 16 segments (as % of cardiac cycle) with a median follow-up duration of 6.3 years (4.46% versus 3.80% with 16% dyssynchrony in CCS) [24]. A study by Yu et al. using the same parameter of dyssynchrony with a median follow up of 7.2 years also found increased dyssynchrony in asymptomatic CCS (7.8% versus 4.9%) [25]. Ylänen et al. performed 3D echocardiography and measured dyssynchrony by calculating the SD of time to reach minimum systolic volume in 12 and in 16 segments rather than using 2DSTE. Follow-up was done in 71 CCS of which eight also received radiotherapy. The longitudinal DI of 16 segments correlated negatively with LVEF. In linear regression a higher DI was correlated with lower LVEF and cardiac irradiation [26].
In contrast with our results, all above authors found dyssynchrony in CCS by using either 3D echocardiography or 2D echocardiography including more segments (2-, 3- and 4-chamber views). Strain as well as volume derived variables have been used in previous research. Insufficient correlation between 2 and 3D echocardiography in assessing dyssynchrony has been reported previously by other authors and could partly explain the difference in results [27, 28].
Myocardial fibrosis can be divided in focal (patchy) fibrosis and diffuse interstitial fibrosis [29]. Detecting patchy fibrosis is done by using Late gadolinium enhancement (LGE) imaging and has been described in studies on anthracycline-induced cardiotoxicity in early- as well as late-onset cardiotoxicity [29, 30]. Diffuse interstitial fibrosis appears to be a more widely reported phenomenon in anthracycline-induced cardiotoxicity and can be measured by quantifying extracellular volume fraction using T1 Mapping [31,32,33]. In more diffuse fibrosis, longitudinal and circumferential dyssynchrony measured with the rough measure of only six segments can be missed.
We found a shorter global T2P systolic LS (uncorrected for heartrate) in survivors compared to controls. We showed a significantly higher heart rate in survivors compared to controls (p = 001). A possible explanation for an increased heart rate might by an increased sympathetic stimulation after anthracycline exposure [34, 35]. Consequently, when corrected for heart rate, the difference in global T2P systolic LS between CCS and controls was diminished (p = 0.335).
Study limitations
Our study has several limitations. First, our pilot study included 115 CCS. A larger population may show statistically significant differences in LVD parameters; however, its clinical relevance should be further investigated. Second, in this study we assessed dyssynchrony using 2DSTE as a quick and easily accessible, bedside method. A more complete assessment of cardiac function using more advanced techniques, e.g., 3D echocardiography or MRI, might be a more sensitive method. Third, we were limited by use of only six segments. Even though this makes our method quickly accessible in children, the use of more segments (e.g., 16 or 18 segments) could be more sensitive in detecting more subtle changes in left ventricular contraction patterns.
Conclusion
In this study, we could not demonstrate LVD in asymptomatic CCS at late follow-up after exposure to anthracyclines when compared to healthy controls. More research needs to be done assessing possible dyssynchrony in a larger population of asymptomatic CCS. We advise using more longitudinal segments and different types of imaging (3D echocardiography and MRI) and compare the results with simultaneous quantification of fibrosis.
Data availability
Upon request.
References
Feijen EA, Font-Gonzalez A, Van der Pal HJ et al (2019) DCOG-LATER study group. Risk and temporal changes of heart failure among 5-year childhood cancer survivors: a DCOG-LATER study. JAHA 8:e009122
Moudgil R, Yeh ET (2016) Mechanisms of cardiotoxicity of cancer chemotherapeutic agents: cardiomyopathy and beyond. Can J Cardiol 32:863–870
Melendez GC, Jordan JH, D’Agostino RB Jr et al (2017) Progressive 3-month increase in LV myocardial ECV after anthracycline-based chemotherapy. JACC Cardiovasc Imaging 10:708–709
Jordan JH, Vasu S, Morgan TM et al (2016) Anthracycline-associated T1 mapping characteristics are elevated independent of the presence of cardiovascular comorbidities in cancer survivors. Circ Cardiovasc Imaging 9:e004325
Nguyen KL, Hu P, Ennis DB et al (2016) Cardiac MRI: a translational imaging tool for characterizing anthracycline-induced myocardial remodeling. Curr Oncol Rep 18:48–72
Neilan TG, Coelho-Filho OR, Shah RV et al (2013) Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracycline-based chemotherapy. Am J Cardiol 111:717–722
Tham EB, Haykowsky MJ, Chow K et al (2013) Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson 10:48–58
Takemura G, Fujiwara H (2007) Doxorubicin cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis 49:330–352
Tigen K, Karaahmet T, Kirma C et al (2010) Diffuse late gadolinium enhancement by cardiovascular magnetic resonance predicts significant intraventricular systolic dyssynchrony in patients with non-ischemic dilated cardiomyopathy. J Am Soc Echocardiogr 23:416–422
Azazy AS, Soliman M, Yaseen R et al (2019) Left ventricular dyssynchrony assessment using tissue synchronization imaging in acute myocardial infarction. Avicenna J Med 9:48–54
Leong DP, Chakrabarty A, Shipp N et al (2012) Effects of myocardial fibrosis and ventricular dyssynchrony on response to therapy in new-presentation idiopathic dilated cardiomyopathy: insights from cardiovascular magnetic resonance and echocardiography. Eur Heart J 33:640–648
Leerink JM, de Baat EC, Feijen EAM et al (2020) Cardiac disease in childhood cancer survivors. Risk prediction, prevention, and surveillance. JACC CardioOncol 2:363–378
Lang RM, Badano LP, Mor-Avo V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update form the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 28:1–39
Rowland DG, Gutgesell HP (1994) Use of mean arterial pressure for noninvasive determination of left ventricular end-systolic wall stress in infants and children. Am J Cardiol 74:98–99
Devereux RB, Reichek N (1977) Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 55:613–618
Pourier MS, Mavinkurve-Groothuis AMC, Dull MM et al (2020) Myocardial 2D strain during long-term (>5 years) follow-up of childhood survivors of acute lymphoblastic leukemia treated with anthracyclines. Am J Cardiol 127:163–168
Klitsie LM, Roest AA, van der Hulst AE et al (2013) Assessment of intraventricular time differences in healthy children using two-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr 26:629–639
Altman DG (1991) Practical statistics for medical research. Chapman and Hall, London
Den Boer SL, du Marchie Sarvaas GJ, Klitsie LM et al (2017) Distribution of strain patterns in children with dilated Cardiomyopathy. Echocardiography 34:881–887
Van der Hulst AE, Delgado V, Blom NA et al (2011) Cardiac resynchronization therapy in paediatric and congenital heart disease patients. Eur Heart J 32:2236–2246
Mele D, Luis GA, Malagù M et al (2018) Echocardiographic evaluation of cardiac dyssynchrony: does it still matter? Echocardiography 35:707–715
Li H, Liu C, Zhang G et al (2019) The early alteration of left ventricular strain and dyssynchrony index in breast cancer patients undergoing anthracycline therapy using layer-specific strain analysis. Echocardiography 36:1675–1681
Okuma H, Noto N, Tanikawa S et al (2017) Impact of persistent left ventricular regional wall motion abnormalities in childhood cancer survivors after anthracycline therapy: assessment of global left ventricular myocardial performance by 3D speckle-tracking echocardiography. J Cardiol 70:396–401
Cheung Y, Hong W, Chan GCF (2010) Left ventricular myocardial deformation and mechanical dyssynchrony in children with normal ventricular shortening fraction after anthracycline therapy. Heart 96:1137–1141
Yu HK, Yu W, Cheuk DK et al (2013) New three-dimensional speckle-tracking echocardiography identifies global impairment of left ventricular mechanics with a high sensitivity in childhood cancer survivors. J Am Soc Echocardiogr 26:846–852
Ylänen K, Eerola A, Vettenranta K et al (2014) Three-dimensional echocardiography and cardiac magnetic resonance imaging in the screening of long-term survivors of childhood cancer after cardiotoxic therapy. Am J Cardiol 113:1886–1892
Bhambhani A, Mathew A (2019) Comparison of three-dimensional echocardiography and speckle tracking echocardiography in quantification and mapping of intraventricular mechanical dyssynchrony. Indian Heart J 71:256–262
Vaidya GN, Salgado BC, Badar F et al (2019) Two-dimensional strain echocardiography-derived left ventricular ejection fraction, volumes, and global systolic dyssynchrony index: comparison with three-dimensional echocardiography. Echocardiography 36:1054–1065
Mewton N, Liu C-Y, Croisille P et al (2011) Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol 57:891–903
Cheung YF, Lam WW, Ip JJ et al (2015) Myocardial iron load and fibrosis in long term survivors of childhood leukemia. Pediatr Blood Cancer 62:698–703
Lunning MA, Kutty S, Rome ET et al (2015) Cardiac magnetic resonance imaging for the assessment of the myocardium after doxorubicin-based chemotherapy. Am J Clin Oncol 38:377–381
Neilan TG, Coelho-Filho OR et al (2013) The myocardial extracellular volume fraction from T1 measurements in healthy volunteers and mice; relationship to aging and cardiac dimensions. JACC Cardiovasc Imaging 6:672–683
Jordan JH, D’Agostino RB Jr, Hamilton CA et al (2014) Longitudinal assessment of concurrent changes in left ventricular ejection fraction and left ventricular myocardial tissue characteristics after administration of cardiotoxic chemotherapies using T1-weighted and T2-weighted cardiovascular magnetic resonance. Circ Cardiovasc Imaging 7:872–879
Nousiainen T, Vanninen E, Jantunen E et al (2001) Neuroendocrine changes during the evolution of doxorubicin-induced left ventricular dysfunction in adult lymphoma patients. Clin Sci (Lond) 101:601–607
Toyoda Y, Okada M, Kashem MA et al (1998) A canine model of dilated cardiomyopathy induced by repetitive intracoronary doxorubicin administration. J Thorac Cardiovasc Surg 115:1367–1373
Acknowledgements
We thank our colleagues from the Late Effects outpatient clinic for their help in obtaining the data.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pourier, M.S., Dull, M.M., Weijers, G. et al. Left ventricular dyssynchrony in long-term childhood cancer survivors treated with anthracyclines: a retrospective cross-sectional study. Int J Cardiovasc Imaging 37, 3469–3475 (2021). https://doi.org/10.1007/s10554-021-02347-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-021-02347-4