Abstract
Prior studies in patients with transcatheter aortic valve implantation (TAVI) demonstrated an influence of transcatheter heart valve (THV) position on the occurrence of new conductions disturbances (CD) and paravalvular leakage (PVL) post TAVI in balloon-expandable valves (BEV). Purpose of this study was to investigate the THV implantation depth and its influence on the occurrence of CD and PVL in self-expanding valves (SEV). We performed fusion imaging of pre- and post-procedural computed tomography angiography in 104 TAVI-patients (all with Evolut R) to receive a 3-D reconstruction of the THV within the native annulus region. The THV length below the native annulus was measured for assessment of implantation depth. Electrocardiograms pre-discharge were assessed for conduction disturbances (CD), PVL was determined in transthoracic echocardiography. The mean implantation depth of the THV in the whole cohort was 4.3 ± 3.0 mm. Using the best cut-off of ≥ 4 mm in receiver operating characteristic curve analysis (sensitivity 83.3%, specificity 60.0%) patients with lower THV position developed more new CD after TAVI (68.2 vs. 23.7%, P < 0.001). A deep THV position was identified as the only predictor for new CD after TAVI (odds ratio [CI] 1.312[1.119–1.539], P = 0.001). The implantation depth showed no influence on the grade of PVL (r = 0.052, P = 0.598). In patients with TAVI using the Evolut R SEV, a lower THV positioning (≥ 4 mm length below annulus) was a predictor for new conduction disturbances. In contrast, implantation depth was not associated with the extent of PVL.
Graphic abstract
Prostheses positions of self-expanding valves and their influence on the occurrence of new conduction disturbances and the grade of paravalvular leakage after TAVI.

Similar content being viewed by others
Introduction
Transcatheter aortic valve implantation (TAVI) is an established treatment alternative to surgical aortic valve replacement in symptomatic aortic stenosis of patients with intermediate to high surgical risk [1]. The most common prosthetic valve types are self-expanding valves (SEV) and balloon-expandable valves (BEV). A large randomized study demonstrated equivalence for both systems with regard to valve-related efficacy endpoints [2]. Yet, a recent study revealed significantly higher rates of paravalvular leakage (PVL) and permanent pacemaker implantation caused by new-onset conduction disturbances (CD) in patients with SEV [3]. These factors are known to be transcatheter heart valve (THV) position related [4,5,6]. A new method of fusion imaging of pre- and post-procedural computed tomography angiography (CTA), published by our group, facilitates a three dimensional visualization of the THV within the native annulus plane after TAVI [7]. Using this method, we revealed a deep implantation of the THV as predictor for new-onset conduction disturbances in patients with BEV [7].
Therefore, we aimed to investigate the THV position of self-expanding valves assessed by fusion imaging method of pre- and post-TAVI CTA and its influence on the occurrence of new CD and PVL.
Methods
Study population
In accordance to the guidelines after thoracic aortic stent implantation a post-TAVI CTA was performed in all patients within our institution [8]. The purpose of this CTA was to identify possible complications, e.g. aortic injuries or thrombosis of the valves. Reasons for not performing a post-procedural CTA were described previously, e.g. renal insufficiency or frailty [9]. All patients with evaluable pre- and post-TAVI CTA and implanted newer generation SEV (Evolut R, Medtronic Inc., Minneapolis, USA) between January 2015 and June 2020 were candidates for study inclusion. Patients with valve-in-valve procedures were excluded. Experienced operators (each with an experience of at least 100 TAVI-procedures) implanted all THVs via a transfemoral access. The multidisciplinary, institutional heart team decided on TAVI eligibility, procedural feasibility as well as the preferred access route or prosthesis type and size [10]. All patients gave written informed consent for TAVI and the anonymized use of clinical, procedural, and follow-up data at the time of the intervention. The study was approved by the local institutional review board and complies with the Declaration of Helsinki.
Image acquisition
Our detailed CTA-protocol was described previously [9]. In brief, we used a second generation dual-source CT scanner (Somatom Definition Flash, Siemens Healthcare, Forchheim, Germany) for the retrospective ECG-gated contrast-enhanced pre- and post-TAVI CTAs (70 mL for pre- and 50 mL for post-TAVI CTA, Imeron 400, Bracco, Konstanz, Germany) [9]. The post-TAVI CTAs were mostly performed between the second and seventh day after the intervention.
We used the “bolus tracking”-technique for beginning the CTA-scans with a region of interest in the left atrium. Images were reconstructed at 50 ms steps throughout the cardiac cycle. The image analysis was conducted by two experienced readers in consensus (P.B. and P.R.) using a post-processing workstation (Syngo Multimodality Workplace, Siemens Healthcare, Forchheim, Germany).
Image analysis
We carried out the measurements of the aortic annulus inclusive the area derived diameter and annulus eccentricity during systole on the pre-TAVI images. Additionally, these sequences were used for a calcification assessment of the device-landing zone. The degree of calcification was visually quantified for each cusp: grade 0: no calcification, grade 1: mild calcification as small calcified spots with minimal diameter ≤ 2 mm, grade 2: moderate calcification as calcified spots with minimal diameter more than 2 mm, grade 3: severe calcification as large calcified formations more than 5 mm minimal diameter [11].
As previously described by our group, fusion imaging of pre- and post-procedural CTA was used for an assessment of the final prosthesis position [7] (Fig. 1). To assess the implantation depth, we measured the THV distance below the native annulus [separately for left coronary cusp (LCC), right coronary cusp (RCC) and non-coronary cusp (NCC)] within the fusion images.
Visualization of fusion imaging process. Pre-TAVI CTA sagittal oblique (a) and axial (b) reconstructions with delineated annulus plane and post-TAVI CTA sagittal oblique reconstruction with the implanted Evolut R (c, * marked a leaflet thrombosis). After semi-automatically merging of the pre- and post-TAVI CTAs (d), we manually adapted the fused images for an optimal alignment of the device-landing zone (e). Finally, the THV distances below the native annulus were measured next to all three cusps to assess the prosthesis implantation depth (f, arrows for distance measurements).—marked the annulus plane
We determined THV tilt in relation to the annulus plane as the arctangent of (maximum − minimum stent center height above the annulus plane adjacent the individual cusps)/mean expanded THV diameter * 180/π) [11]. The THV area measurements of the stent center and the left ventricular outflow tract end (LVOT) were used to calculate the prosthesis expansion differentiated for both heights as (measured THV area/manufacturer reported THV area for the respective height) × 100 (Fig. 2).
Determination of prosthesis expansion. Pre-TAVI CTA sagittal oblique (a, — marked the annulus plane, - - marked the heights of THV area measurements) and axial (b,c) reconstructions for measurement of the prosthesis area on the height of the THV center (b) and at the LVOT end (c). The measured values are set in relation to the manufacturer reported ones to determine the prosthesis expansion
Assessment of paravalvular leakage
Experienced operators examined the amount of PVL in transthoracic echocardiography before discharge. Thereby, the PVL was visually graduated in none, trivial, mild, moderate and severe.
Electrocardiogram monitoring
All patients remained on telemetric monitoring for a minimum of 48 h post-intervention. Furthermore, a twelve-lead electrocardiogram (ECG) was obtained in every patient pre-TAVI, daily in the initial two days after procedure and thereafter all two days until discharge. We added a 24-h Holter-ECG in patients with any conduction disturbances (CD) on twelve-lead-ECG or during monitoring. New CD after TAVI were defined as new onset of any kind of atrioventricular block or bundle branch block—if they persisted until discharge. A progress of an atrioventricular or a new bundle branch block was determined as CD in case of preexisting conduction disturbances. Patients with implanted pacemaker prior to TAVI were excluded from the subanalysis of predictors for CD after TAVI.
Statistical analysis
We used SPSS software, Version 25.0 (IBM Corp., Armonk, NY, USA) and MedCalc, Version 19.4 (MedCalc Software Ltd., Ostend, Belgium) for the statistical analyzes. Categorical data are depicted as frequencies and percentages, continuous variables as mean with standard deviation or median with interquartile range. To test differences between two groups (e.g. between patients with and without CD) we used the χ2-test (for categorical variables), the Student`s t-test (for normal distributed continuous variables) or the Mann–Whitney-U Test (non-normal distributed continuous variables). Normal distribution was examined by the Kolmogorov–Smirnov-Test. To test the linear correlation between the implantation depth or annular/THV eccentricity and PVL we used the Spearman´s rank correlation. We used univariate and multivariate logistic regression models to assess possible predictors for new CD, a lower prosthesis position or LT. The multivariate models examined variables with a P-value < 0.1 in univariate analysis. A P value of < 0.05 was defined as statistically significant in all tests.
Results
During the study period 118 patients with an implanted Evolut R THV received a post-TAVI CTA. The image quality of two post-TAVI CTAs was too poor to perform fusion imaging. Ten patients were excluded due to a valve-in-valve procedure and two patients received a surgical revision caused by a THV dislocation.
The mean age of the study cohort of 104 patients (66.3% female) was 82.2 ± 5.2 years with a mean logistic Euroscore of 15.1 ± 11.3%. Preexisting CD were documented in 48 patients (46.2%), five patients had a permanent pacemaker before TAVI.
The mean implantation depth of the THV in the whole cohort was 4.3 ± 3.0 mm below the annulus plane. There were significant differences in implantation depth when analyzing individual cusps separately with the right and left coronary cusp showing lower positions compared to the non-coronary cusp (4.9 ± 2.8 mm and 4.9 ± 3.4 mm vs. 3.1 ± 3.5 mm, P < 0.001).
All baseline, procedural and prosthesis-related characteristics are presented in Table 1.
New conduction disturbances
Ninety-nine pacemaker naïve patients were monitored for CD post-TAVI. New onset CD developed in 54 (54.5%) of these patients.
Among the patients without preexisting CD 10 exhibited a left bundle branch block (LBBB) or a first and third degree atrioventricular block (each six patients). Of the patients with preexisting CD each four patients with first degree atrioventricular block developed a third degree atrioventricular block or a new LBBB (Table 2).
Patients of both groups were comparable for baseline and procedural characteristics (Table 3). Patients with new CD revealed a significant lower mean stent position (5.2 ± 2.4 mm vs. 3.1 ± 3.3 mm, P < 0.001). This was also observed in the subanalysis of all three cusps (P < 0.001 for LCC and RCC, P = 0.004 for NCC). However, the grade of calcification of the device-landing zone was similar between the two groups—both in total and adjacent to the LCC, RCC or NCC (P = 0.438, P = 0.993, P = 0.650 and P = 0.140, respectively). Twenty-five patients (25.3%) received a permanent pacemaker implantation after TAVI.
We included the age, logistic Euroscore, preexisting CD, total grade of calcification of the device-landing zone, prosthesis size, postdilatation, THV expansion of the stent center and the LVOT end as well as the mean implantation depth below annulus in the logistic regression models. After multivariate adjustment only the mean implantation depth was identified as predictor for new CD after TAVI (odds ratio [CI] 1.312[1.119–1.539], P = 0.001) (Table 4).
Lower implantation depth
In the receiver operating characteristic curve analysis, a mean implantation depth of ≥ 4 mm demonstrated the best cut-off for occurrence of new CD post-TAVI (Sensitivity 83.3%, Specificity 60.0%). Therefore, we defined a lower implantation depth as at least 4 mm of the prosthesis below (ventricular) the native annulus plane. THVs were found in 66 patients (63.5%) in a lower and in 38 patients (36.5%) in a higher position. Using this cut off patients with a lower THV position developed more new CD after TAVI (68.2 vs. 23.7%, P < 0.001) (Table 3). Patients with a lower implantation depth were less often women (57.6 vs. 81.6%, P = 0.013), had a larger annulus diameter (23.6 ± 2.2 vs. 22.3 ± 2.1 mm, P = 0.002) and we found a significant difference for implanted valve sizes (P = 0.006). There was a trend for a higher calcification grade of the left coronary cusp (1.5 ± 0.5 vs. 1.3 ± 0.5 mm, P = 0.060).
Additionally, a lower prosthesis position is associated with a larger THV expansion at the LVOT end (67.3 ± 11.8 vs. 55.1 ± 8.0%, P < 0.001).
Predictors for a lower THV position
We included gender, annulus diameter, prosthesis size and the calcification grade of the left coronary cusp in the logistic regression models. After multivariate adjustment, none of the variables were significantly predictive for a lower THV position (Data not shown).
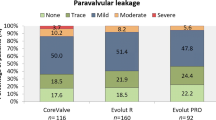
Paravalvular leakage
PVL was classified as none in 34 (32.7%), trivial in 25 (24.0%), mild in 40 (38.5%) and moderate in 5 (4.8%) patients (shown in Table 1). A lower THV position had no influence on the grade of PVL as shown by the Spearman´s rank correlation between the mean implantation depth and PVL (r = 0.052, P = 0.598, data not shown). Furthermore, neither the eccentricity of the native annular plane (r = 0.020, P = 0.841) nor of the deposited prosthesis on this height (r = 0.113, P = 0.255) were predictive for PVL. However, the grade of calcification has an influence on the grade of PVL (r = 0.298, P = 0.002).
Discussion
In this comprehensive analysis using 3D fusion imaging of pre-and post-TAVI CTA we investigated the positioning of self-expandable THVs. In patients with TAVI using the Evolut R device a lower THV positioning is a predictor for new conduction disturbances. However, we could not identify any factors that predispose to low positioning. Implantation depth was not associated with the grade of PVL.
New conduction disturbances after TAVI
After TAVI using SEV, 54.5% of the patients developed new CD, 25.3% received a permanent pacemaker implantation. These complications have potential effects on long-term outcomes as well as enormous influence on health economics [12]. Therefore, the identification of potential predictors for conduction disturbances is crucial.
In this study, a lower mean implantation depth was the only predictor for new CD using fusion imaging for a reliable 3-D depiction of the THV within the native annulus region. This results are in line with preexisting data for various valve types, where a deep prosthesis position in the LVOT, though only assessed in fluoroscopy or post-TAVI CTA, is a risk factor for CD [4, 5]. A mean implantation depth of ≥ 4 mm demonstrated the best cut-off for occurrence of new CD post-TAVI in our study. This value, measured by 3D fusion imaging, is in line with the results of the ADVANCE II trial, in which a prosthesis position shallower than 4 mm in the LVOT was associated with less permanent pacemaker implantations [13]. However, in ADVANCE II, the 2D angiographic depiction of the annulus is limited by its two-dimensionality and may be potentially misleading due to the complex geometry of the aortic annulus [7]. The manufacturer recommended a final implantation position with 3 to 5 mm of the prosthesis length below annular plane.
In contrast to previous studies investigating BEV, the calcification burden of the device landing zone had no influence on the occurrence of new CD [7, 14]. One might assume that through the self-expandable mechanism calcifications spots were pushed less into the surrounding tissue. Therefore, the resulting tissue damage of the conduction system in close proximity to the subaortic region and membranous septum seems to be lower.
Low prosthesis position
Based on fusion imaging of pre- and post-TAVI CTA none of the structural or procedural related criteria revealed to be predictive for a low prosthesis position in SEV. In the light of a correlation of lower implantation depth and the occurrence of CD, a higher deposition of the prosthesis should be considered in all patients with pre-existing CD to avoid new CD. A former study of our group determined a reduced calcium burden within the cusp region as the sole independent predictor for a low position in patients with BEV [11].
Paravalvular leakage
The geometric specialty of the Evolut R is a highest radial force at the LVOT edge of the prosthesis and a continuously decreasing diameter towards the stent center. With respect to this, a recently published study identified the implantation depth as predictor for moderate or severe PVL [15]. Our data showed no influence of the implantation depth or the eccentricity of the annular plane (native or the prosthesis on this height) on PVL. This could be explained by the fact that the mean implantation depth in our study was higher and more following the manufactures recommendation than in the above study (4.3 ± 3.0 mm vs. 6.2 ± 2.9 mm). According to previous publications, a higher calcification grade of the device landing zone is associated with a more pronounced PVL [16, 17].
In summary, based on our results of the correlation between the implantation depth a high THV deposition might be desirable to avoid pacemaker implantations. However, high positioning carries the risk of complete THV dislocation into the ascending aorta. Therefore, the implantation depth should be aimed at the lower range of the manufacturer recommendation with around 3 mm prosthesis length below the annular plane.
Limitations
The limited sample size of our cohort limits the power to identify minor predictors for the occurrence of new CD or a lower prosthesis position. Especially the prevalence of right bundle branch block is low, what is a known preditor of worsening post TAVI conduction disturbances. While a lower mean implantation depth was a predictor for new CD, there was only a trend towards a higher PM implantation rate in these patients. A larger patient cohort might help to clarify the clinical significance of these CD. Moreover, our study was limited to patients with one specific SEV type. It remains speculative, if these results are transferable to other SEV types.
Conclusion
In patients with TAVI using the Evolut R SEV, a lower THV positioning (≥ 4 mm length below annulus) was a predictor for new conduction disturbances. In contrast, implantation depth was not associated with the extent of PVL.
Clinical perspectives
In the light of a further expansion of TAVI-procedure, an increased knowledge about the underlying mechanisms of possible complications is crucial for any specific valve design. Our study suggests that an implantation depth of ≥ 4 mm of the Evolut R leads to significantly more new CD after TAVI, whereas a higher position is not associated with more PVL. With respect to this, a deposition of the prosthesis above this value should be considered. Structural characteristics of the annular region showed no independent influence on implantation depth.
Abbreviations
- BEV:
-
Balloon-expandable valves
- CD:
-
Conduction disturbances
- CTA:
-
Computed tomography angiography
- ECG:
-
Electrocardiogram
- LVOT:
-
Left ventricular outflow tract
- PVL:
-
Paravalvular leakage
- SEV:
-
Self-expanding valves
- TAVI:
-
Transcatheter aortic valve implantation
- THV:
-
Transcatheter heart valve
References
Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Munoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL (2017) 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 38:2739–2791. https://doi.org/10.1093/eurheartj/ehx391
Thiele H, Kurz T, Feistritzer HJ, Stachel G, Hartung P, Eitel I, Marquetand C, Nef H, Doerr O, Lauten A, Landmesser U, Abdel-Wahab M, Sandri M, Holzhey D, Borger M, Ince H, Öner A, Meyer-Saraei R, Wienbergen H, Fach A, Frey N, König IR, Vonthein R, Rückert Y, Funkat AK, de Waha-Thiele S, Desch S (2020) Comparison of newer generation self-expandable vs. balloon-expandable valves in transcatheter aortic valve implantation: the randomized SOLVE-TAVI trial. Eur Heart J 41:1890–1899. https://doi.org/10.1093/eurheartj/ehaa036
Elgendy IY, Gad MM, Mahmoud AN, Dvir D, Kapadia SR, Alfonso F, Capodanno D (2020) Meta-analysis comparing outcomes of self-expanding versus balloon-expandable valves for transcatheter aortic valve implantation. Am J Cardiol 128:202–209. https://doi.org/10.1016/j.amjcard.2020.05.007
Schwerg M, Fulde F, Dreger H, Poller WC, Stangl K, Laule M (2016) Optimized implantation height of the Edwards SAPIEN 3 valve to minimize pacemaker implantation after TAVI. J Interv Cardiol 29:370–374. https://doi.org/10.1111/joic.12302
Fraccaro C, Buja G, Tarantini G, Gasparetto V, Leoni L, Razzolini R, Corrado D, Bonato R, Basso C, Thiene G, Gerosa G, Isabella G, Iliceto S, Napodano M (2011) Incidence, predictors, and outcome of conduction disorders after transcatheter self-expandable aortic valve implantation. Am J Cardiol 107:747–754. https://doi.org/10.1016/j.amjcard.2010.10.054
Sherif MA, Abdel-Wahab M, Stöcker B, Geist V, Richardt D, Tölg G, Richardt G (2010) Anatomic and procedural predictors of paravalvular aortic regurgitation after implantation of the medtronic corevalve bioprosthesis. J Am Coll Cardiol 56:1623–1629. https://doi.org/10.1016/j.jacc.2010.06.035
Ruile P, Pache G, Minners J, Hein M, Neumann FJ, Breitbart P (2019) Fusion imaging of pre- and post-procedural computed tomography angiography in transcatheter aortic valve implantation patients: evaluation of prosthesis position and its influence on new conduction disturbances. Eur Heart J Cardiovasc Imaging 20:781–788. https://doi.org/10.1093/ehjci/jey195
Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, Kouchoukos NT, Lytle BW, Milewicz DM, Reich DL, Sen S, Shinn JA, Svensson LG, Williams DM (2010) 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 121:e266–e369. https://doi.org/10.1161/CIR.0b013e3181d4739e
Pache G, Schoechlin S, Blanke P, Dorfs S, Jander N, Arepalli CD, Gick M, Buettner HJ, Leipsic J, Langer M, Neumann FJ, Ruile P (2016) Early hypo-attenuated leaflet thickening in balloon-expandable transcatheter aortic heart valves. Eur Heart J 37:2263–2271. https://doi.org/10.1093/eurheartj/ehv526
von Scheidt W, Welz A, Pauschinger M et al (2020) Interdisciplinary consensus on indications for transfemoral transcatheter aortic valve implantation (TF-TAVI): Joint Consensus Document of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte e.V. (ALKK) and cooperating Cardiac Surgery Departments. Clin Res Cardiol 109:1–12. https://doi.org/10.1007/s00392-019-01528-5
Breitbart P, Pache G, Minners J, Hein M, Schröfel H, Neumann FJ, Ruile P (2020) Predictors for low TAVI-prosthesis position assessed by fusion imaging of pre- and post-procedural CT angiography. Clin Res Cardiol. https://doi.org/10.1007/s00392-020-01654-5
Dizon JM, Nazif TM, Hess PL, Biviano A, Garan H, Douglas PS, Kapadia S, Babaliaros V, Herrmann HC, Szeto WY, Jilaihawi H, Fearon WF, Tuzcu EM, Pichard AD, Makkar R, Williams M, Hahn RT, Xu K, Smith CR, Leon MB, Kodali SK (2015) Chronic pacing and adverse outcomes after transcatheter aortic valve implantation. Heart 101:1665–1671. https://doi.org/10.1136/heartjnl-2015-307666
Petronio AS, Sinning JM, Van Mieghem N, Zucchelli G, Nickenig G, Bekeredjian R, Bosmans J, Bedogni F, Branny M, Stangl K, Kovac J, Schiltgen M, Kraus S, de Jaegere P (2015) Minimizing permanent pacemaker following repositionable self-expanding transcatheter aortic valve replacement. JACC Cardiovasc Interv 8:837–846. https://doi.org/10.1016/j.jcin.2015.02.005
Mauri V, Reimann A, Stern D, Scherner M, Kuhn E, Rudolph V, Rosenkranz S, Eghbalzadeh K, Friedrichs K, Wahlers T, Baldus S, Madershahian N, Rudolph TK (2016) Predictors of permanent pacemaker implantation after transcatheter aortic valve replacement with the SAPIEN 3. JACC Cardiovasc Interv 9:2200–2209. https://doi.org/10.1016/j.jcin.2016.08.034
Gorla R, De Marco F, Garatti A, Bianchi G, Popolo Rubbio A, Acerbi E, Casenghi M, Spagnolo P, Brambilla N, Testa L, Agnifili ML, Tusa M, Bedogni F (2020) Impact of aortic angle on transcatheter aortic valve implantation outcome with Evolut-R, Portico, and Acurate-NEO. Catheter Cardiovasc Interv. https://doi.org/10.1002/ccd.28957
Marwan M, Achenbach S, Ensminger SM, Pflederer T, Ropers D, Ludwig J, Weyand M, Daniel WG, Arnold M (2013) CT predictors of post-procedural aortic regurgitation in patients referred for transcatheter aortic valve implantation: an analysis of 105 patients. Int J Cardiovasc Imaging 29:1191–1198. https://doi.org/10.1007/s10554-013-0197-7
Hagar A, Li Y, Wei X, Peng Y, Xu Y, Ou Y, Wang Z, Wang X, Shah JP, Sihag V, Chen M, Feng Y (2020) Incidence, predictors, and outcome of paravalvular leak after transcatheter aortic valve implantation. J Interv Cardiol. https://doi.org/10.1155/2020/8249497
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
PB (corresponding author) conceived and performed the analysis and interpretation of data, drafted the manuscript and approved the submission. JM and HS revised the manuscript critically for important intellectual content. MH performed analysis and interpretation of data. FN revised the manuscript critically for important intellectual content and approved finally the submission. PR conceived the conception and design of the study, performed analysis and interpretation of data, drafted the manuscript and approved the submission.
Corresponding author
Ethics declarations
Conflict of interest
Franz-Josef Neumann reports that his institution has received research grants, consultancy fees, and speaker honoraria from Daiichi Sankyo, Astra Zeneca, Sanofi-Aventis, Bayer, The Medicines Company, Bristol, Novartis, Roche, Boston Scientific, Biotronik, Medtronic, Edwards and Ferrer. Holger Schröfel is proctor for Edwards, Medtronic and Boston Scientific. The remaining authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Breitbart, P., Minners, J., Hein, M. et al. Implantation depth and its influence on complications after TAVI with self-expanding valves. Int J Cardiovasc Imaging 37, 3081–3092 (2021). https://doi.org/10.1007/s10554-021-02275-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-021-02275-3