Abstract
Low-dose dobutamine stress echocardiography (DSE) is a valuable tool to distinguish true-severe (TS) from pseudo-severe (PS) low gradient aortic valve stenosis (LGAS) in patients with reduced left ventricular ejection fraction (LVEF). However, only scanty studies reported the clinical utility of DSE in differentiating TS-LGAS patients with preserved LVEF. We investigated the clinical utility of DSE in LGAS patients with preserved LVEF and the echocardiographic determinants suggestive of TS-LGAS. 130 consecutive LGAS patients [indexed aortic valve area (AVA) ≤ 0.6cm2/m2 and mean trans-aortic pressure gradient (PGmean) < 40mmHg] with preserved (≥ 50%, n = 63) and reduced (< 50%, n = 67) LVEF were included. DSE defined TS-LGAS (projected AVA ≤ 1 cm2) in 61.2% patients with reduced LVEF and in 68.3% patients with preserved LVEF. Multivariate logistic regression analysis showed that baseline AVA was an independent determinant of TS-LGAS both in LVEF ≥ 50% (OR 0.45, P = 0.004) and LVEF < 50% groups (OR 0.55, P = 0.005). Reduced septal and lateral mitral annular plane systolic excursion (MAPSE, OR 0.72 and 0.75, P = 0.013 and 0.016) and septal TDI-s´ were significantly associated with TS-LGAS in patients with LVEF ≥ 50%. Higher systolic pulmonary artery pressure (SPAP, OR 1.43, P = 0.045) was associated with TS-LGAS in patients with LVEF < 50%. DSE is useful to define TS-LGAS also in patients with preserved LVEF. Lower baseline AVA values are linked with TS-LGAS in both patients with reduced and preserved LVEF. Reduced MAPSE and septal TDI-s´ are suggestive of TS-LGAS in patients with preserved LVEF, while higher SPAP is associated with TS-LGAS in patients with reduced LVEF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
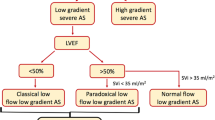
Severe aortic stenosis (AS) is usually defined as an aortic valve area (AVA) < 1.0cm2, mean transvalvular gradient (PGmean) ≥ 40 mmHg, and a peak flow velocity ≥ 4.0 m/s [1]. Patients with AVA < 1.0cm2 and PGmean<40 mmHg are usually defined as low-gradient AS (LGAS). Further examinations are usually required to distinguish true-severe (TS) from pseudo-severe (PS) AS, which is clinically essential for therapeutic decision making because patients with TS-LGAS might benefit, whereas PS-LGAS patients might not benefit from aortic valve replacement (AVR) [2]. LGAS is usually found in patients with reduced LV ejection fraction (LVEF < 50%), but might also be present in patients with preserved LVEF ≥ 50% (so called paradoxical LGAS) [3]. Low-dose dobutamine stress echocardiography (DSE) is recommended as a tool to define the severity of aortic stenosis in patients with LGAS and reduced LVEF [4, 5]. To date, data regarding the utility of DSE for defining severity of LGAS in patients with preserved LVEF presenting with either paradoxical low flow or normal flow remain scanty. In the present study, we observed if DSE could also be useful to define AS severity in LGAS patients with preserved LVEF. We further explored the conventional echocardiographic markers suggestive of TS-LGAS in LGAS patients with reduced or preserved LVEF.
Methods
Study population
A total of 130 consecutive symptomatic low-gradient AS patients (aged 78 ± 8 years, 63.8% male), referred to the University Hospital Würzburg between January 2011 and December 2016, were included in this study. All patients underwent both standard transthoracic echocardiogram and DSE. Enrollment criteria included indexed aortic valve area (AVAi) ≤ 0.6 cm2/m2 and mean trans-aortic pressure gradient (PGmean) < 40 mmHg as assessed by transthoracic echocardiogram. The study protocol is shown in Fig. 1. The study was conducted in accordance to the Declaration of Helsinki and was approved by the Local Ethics Committee at the University of Würzburg (AZ 11/03 and 60/14). Informed consent was obtained from all patients or their guardians.
Echocardiography
Echocardiographic examination was performed with GE Vingmed Vivid 7 or E9, Horten, Norway. Standard measurements on cardiac structural and functional parameters were made according to the current ASE guideline [6]. LV mass indexed to body surface area (LVMi) was calculated by the suggested formula [7]. End-diastolic and end-systolic volumes and LVEF were measured with the biplane Simpson method in the apical 4- and 2-chamber views. Septal and lateral mitral annular plane systolic excursion (MAPSE) and tricuspid annular plane systolic excursion (TAPSE) were measured by M-mode in the apical 4-chamber view. Systolic pulmonary artery pressure (SPAP) was derived from peak tricuspid regurgitation jet velocity using the simplified Bernoulli equation, in combination with the estimated right atrial pressure. LV diastolic function was assessed according to ASE guidelines for the assessment of diastolic function [8]. Tissue Doppler imaging was performed at the septal and lateral mitral annular sites enabling on-line derivation of myocardial systolic velocity (TDI-s′) and diastolic early velocity (e′) [9]. Speckle tracking imaging analysis was performed using EchoPAC software (GE, Horten, Norway) as described previously [10]. Longitudinal peak systolic strain (LS) of each segment was measured and global LS values were acquired by averaging strain rate and strain values of all 18 segments.
Evaluation of AS severity by standard echocardiography
The diagnosis and classification of AS was made according to recent EAE/ASE recommendations [11]. Left ventricular outflow tract (LVOT) diameter was measured in the parasternal long-axis view focusing on the LVOT at baseline, and this value was also used to calculate the aortic valve area during DSE. The outer edge of the velocity spectrum obtained by continuous wave (CW) Doppler across the aortic valve (AV) was traced to obtain the maximum velocity (Vmax), maximum and mean trans-aortic pressure gradient (PGmean and PGmax), and AV velocity time integral (VTI). The subvalvular Vmax (LVOT Vmax), VTI and stroke volume (SV) were obtained by tracing the outer edge of the velocity spectrum at the LVOT by the pulsed wave (PW) Doppler. Aortic valve area by continuity equation VTI [AVA (VTI)] was automatically calculated using the formula:
Valvuloarterial impedance (Zva) was calculated using the formula:
Low-dose dobutamine stress echocardiography
Dobutamine infusion was started at a dose of 5 µg/kg/min, then increasing to 10, 15, and 20 µg/kg/min at 3-min intervals. Blood pressure and heart rate were recorded at rest and at the end of each stage of dobutamine dosage. Contractile reserve was defined as an increase in stroke volume (SV) of 20% or more [12]. The projected aortic valve area at normal transvalvular flow rate (AVAproj) was calculated using a simplified method with the following formula [13]: Simplified AVAproj = AVArest + VCsimpl × (250-Qrest), where VCsimpl is the valve compliance and Q was computed by dividing SV by LV ejection time. VCsimpl was computed with the formula:
where AVArest and AVApeak are AVA at rest and peak DSE. Qpeak and Qrest are Q at rest and peak DSE. TS-LGAS was defined as AVAproj ≤1 cm2.
Follow-up
Patients were followed up for a mean of 18 (12–27) months by clinical visit or telephone interview.
Data analysis
Continuous variables are presented as mean ± standard deviation or median (interquartile range, IQR), as appropriate. Normal distribution of all continuous variables was tested by inspecting skewness, kurtosis, and Q–Q plots. Clinical and echocardiographic continuous variables between preserved and reduced LVEF groups and between PS-LGAS and TS-LGAS groups were compared using independent Student´s t test or Mann–Whitney U test as indicated. Categorical variables were expressed as percentages and were compared using a Chi square test or Fisher’s exact test, as appropriate. Multivariate logistic regression analysis was conducted to identify markers for differentiating TS-LGAS from PS-LGAS. Odds ratios (OR) with 95% confidence interval (CI) were assessed for indicating diagnostic performance. A two-tailed probability value < 0.05 was considered significant. Statistical analysis was performed using IBM SPSS, version 23 for Windows (IBM Corp., New York, USA).
Results
Clinical characteristics
Patients were divided into two subgroups: LVEF ≥ 50% group (n = 63) and LVEF < 50% group (n = 67). As shown in Table 1, the proportion of male patients was significantly higher in the LVEF < 50% group than in the LVEF ≥ 50% group (82.1% vs. 44.4%, P < 0.001). Prevalence of NYHA class III-IV was documented in 79.1% of LVEF < 50% patients and 55.5% of LVEF ≥ 50% patients (P = 0.003). The prevalence of diabetes (52.2% vs. 33.3%, P = 0.030) and coronary heart disease (71.6% vs. 49.2%, P = 0.009) was also significantly higher in the LVEF < 50% group than in the LVEF ≥ 50% group.
Baseline echocardiographic characteristics
Mean LVEF was 60 ± 6% in the LVEF ≥ 50% group and 46 ± 4% in the LVEF < 50% group (Table 2). Patients with LVEF < 50% had larger LV, RV and LA cavities, thicker LV walls, lower septal and lateral MAPSE and TAPSE as compared to patients with LVEF ≥ 50% (all P < 0.05). AV Vmax, PGmean, LVOT Vmax, AV velocity ratio, and SV were significantly lower, while Zva was significantly higher in the LVEF < 50% group than in the LVEF ≥ 50% group (all P < 0.05). AVA VTI was similar between the LVEF LVEF ≥ 50% and < 50% groups (0.86 ± 0.14 vs. 0.84 ± 0.16cm2, P = 0.538). LV longitudinal systolic function parameters including septal and lateral TDI-s´as well as global and regional longitudinal strain (LS) at the basal septum were significantly lower in the LVEF < 50% group than that in the LVEF ≥ 50% group.
DSE
DSE results in the patients with LVEF ≥ 50% and LVEF < 50% are shown in Tables 3 and 4. Forty-three out of 63 (68.3%) in the LVEF ≥ 50% group and 41 out of 67 (61.2%) patients in the LVEF < 50% group were diagnosed as TS-LGAS by DSE. AV Vmax, PGmean, LVOT Vmax, AV velocity ratio, and AVA VTI significantly increased during DSE both in LVEF ≥ 50% and < 50% groups (all P < 0.05, Tables 3, 4). Systolic blood pressure remained unchanged while peak diastolic blood pressure decreased in both groups during DSE.
In the LVEF ≥ 50% group, baseline AVA_VTI (0.86 ± 0.14 vs. 0.97 ± 0.14 cm2, P = 0.007) and baseline MAPSE (septal: 7.9 ± 2.2 vs. 9.5 ± 2.2 mm, P = 0.009; lateral: 9.8 ± 2.6 vs. 11.6 ± 2.2 mm, P = 0.011) were significantly lower in the TS-LGAS subgroup than in the PS-LGAS subgroup. During DSE, AV velocity ratio was significantly increased in the PS-LGAS group (baseline 0.25 ± 0.04 vs. peak 0.29 ± 0.05, P < 0.001), while remained unchanged in the TS-LGAS group (baseline 0.23 ± 0.04 vs. peak 0.24 ± 0.05, P = 0.531). AVA_VTI was significantly increased during DSE in the PS-LGAS group (baseline 0.97 ± 0.14 vs. peak 1.23 ± 0.24 cm2, P < 0.001) and in the TS-LGAS group (baseline 0.86 ± 0.14 vs. peak 0.96 ± 0.20 cm2, P = 0.001).
In the LVEF < 50% group, baseline AV velocity ratio (0.19 ± 0.04 vs. 0.22 ± 0.03, P = 0.006) and baseline AVA_VTI (0.81 ± 0.18 vs. 0.94 ± 0.22 cm2, P = 0.008) were significantly lower, while baseline SPAP (46 ± 15 vs. 37 ± 14 mmHg, P = 0.031) was significantly higher in the TS-LGAS group than in the PS-LGAS group. AV velocity ratio (baseline 0.22 ± 0.03 vs. peak 0.26 ± 0.07, P = 0.001) and AVA_VTI (baseline 0.94 ± 0.22 vs. peak 1.12 ± 0.28 cm2, P = 0.005) were significantly increased in the PS-LGAS group, while remained unchanged in the TS-LGAS group during DSE (AV velocity ratio: baseline 0.19 ± 0.04 vs. peak 0.19 ± 0.05, P = 0.352; AVA_VTI: baseline 0.81 ± 0.18 vs. peak 0.80 ± 0.19 cm2, P = 0.742).
All patients with baseline AVAi ≤ 0.3cm2/m2 were identified as TS-LGAS by DSE (n = 5). In the LVEF ≥ 50% group, 100%, 65.6% and 57.1% of patients with a baseline AVAi of 0.31–0.4, 0.41–0.5 and 0.51–0.6cm2/m2 were identified as TS-LGAS, respectively. In the LVEF < 50% group, 78.9%, 65.5%, and 25.0% of patients with a baseline AVAi of 0.31–0.4, 0.41–0.5, and 0.51–0.6cm2/m2 were identified as TS-LGAS, respectively. Baseline AVAi ≤ 0.4cm2/m2 was highly suggestive of TS-LGAS in LGAS patients with LVEF ≥ 50% (specificity 100% and sensitivity 23%).
Significant mitral annular calcification (MAC) was found in 80 out of 130 (61.5%) patients. As shown in Fig. 2a, baseline septal MAPSE was significantly lower in the TS-LGAS group as compared to the PS-LGAS group in LVEF ≥ 50% patients without significant MAC (8.3 ± 2.1 vs. 10.5 ± 2.0 mm, P = 0.005). Lateral MAPSE was significantly lower in TS-LGAS group as compared to PS-LGAS group in LVEF ≥ 50% patients without (10.7 ± 2.4 vs. 12.3 ± 1.9 mm, P = 0.042) and with significant MAC (8.4 ± 2.2 vs. 10.5 ± 2.3 mm, P = 0.048; Fig. 2b).
Echocardiographic markers suggestive of TS-LGAS
Parameters, which are significantly associated with TS-LGAS with a statistic difference (p < 0.05) between the PS-LGAS and TS-LGAS groups either in the subgroup of LVEF ≥ 50% or in the subgroup of LVEF < 50% (Tables 4, 5), were tested in the multivariable binary regression models. Multivariable logical regression models (Table 6) showed that baseline AVA_VTI was an independent determinant of TS-LGAS both in the LVEF ≥ 50% and < 50% groups after adjusted for age and sex (LVEF ≥ 50%: OR 0.45, P = 0.004; LVEF < 50%: OR 0.55, P = 0.005). Besides, lower septal and lateral MAPSE (adjusted OR 0.72–0.75, P = 0.013–0.016) as well as septal TDI-s´ (adjusted OR 0.53, P = 0.010) were significantly associated with TS-LGAS in patients with LVEF ≥ 50%. Higher SPAP and lower AV velocity ratio were associated with TS-LGAS in patients with LVEF < 50% (SPAP: OR 1.43, P = 0.045; AV velocity ratio: OR 0.21, P = 0.035).
Clinical Follow up results
During follow-up, 28 (21.5%) patients died, 10 (15.9%) in LVEF ≥ 50% group [3 with TS-LGAS (conservative therapy) and 2, 3, 7 with PS-LGAS (2 with conservative therapy, 3 surgical aortic valve replacement, and 2 transcatheter aortic valve implantation)], and 18 (26.9%) in LVEF < 50% group [10 with TS-LGAS (6 conservative therapy, 1, 3, 8 surgical aortic valve replacement, and 3 transcatheter aortic valve implantation) and 8 with PS-LGAS (6 with conservative therapy, 1 surgical aortic valve replacement, and 1 transcatheter aortic valve implantation)].
Discussion
The major findings of the present study are: (1) DSE appears to also be a helpful tool of defining true-severe low gradient aortic stenosis in patients with LVEF ≥ 50%; (2) Baseline AVA derived from transthoracic echocardiography is an independent determinant of TS-LGAS in both patients with LVEF ≥ 50% as well as LVEF < 50%; (3) Reduced septal and lateral MAPSE as well as septal TDI-s´ are associated with TS-LGAS in patients with LVEF ≥ 50% while higher SPAP and lower AV velocity ratio are independently associated with TS-LGAS in patients with LVEF < 50%.
Value of DSE in LGAS patients with reduced and preserved LVEF
Patients with low gradient severe aortic stenosis (LGAS) and preserved LVEF (AVA <1 cm2, PGmean <40 mmHg, LVEF >50%) are increasingly recognised in clinical practice. DSE is recommended to define the severity of aortic stenosis in patients with LGAS and reduced LVEF [4, 5]. Our results showed that DES is also useful in defining TS- from PS-LGAS in patients with LVEF < 50% (Table 4). This finding is in line with a recent study, which demonstrated that DSE might serve as a valuable tool to distinguish TS-LGAS from PS-LGAS in patients with paradoxical LGAS [14].
There were 20 out of 63 (31.7%) LGAS patients with preserved LVEF presenting with paradoxical low-flow LGAS (SVi ≤ 35 ml/m2) in our cohort. We compared other related parameters as well as the response on DSE between the two subgroups (low-flow vs. normal-flow LGAS). The data show that LV cavity in these patients was smaller than in patients with normal-flow LGAS (left ventricular end-diastolic dimension 43 ± 4 vs. 46 ± 5 mm, P = 0.021). Moreover, DSE seems also to be feasible to identify the TS-AS patients in the low-flow LGAS subgroup as in the normal-flow LGAS subgroup (Supplementary Table 1). Future studies with larger patient cohort are warrant to verify these results.
Echocardiographic determinant of TS-LGAS in patients with preserved LVEF
In line with previous findings from our group [15] and others [16], the present study results demonstrate that reduced MAPSE is suggestive of TS-LGAS in patients with LVEF > 50%. A previous study also showed that MAPSE correlated with aortic valve area (Spearman r = 0.18, P = 0.02) in 205 asymptomatic AS patients with LVEF ≥ 50% [17]. In another study, Rydberg and colleagues found that left atrioventricular plane displacement (MAPSE), but not left ventricular ejection fraction, was influenced by the degree of aortic stenosis [16]. In patients with LGAS and preserved LVEF, LVEF is maintained at normal range at the expense of high LV end-diastolic pressure and volume. In this circumference, LV cannot recruit additional preload because the maximum sarcomere length is already reached and the hypertrophied stiff ventricle could not tolerate additional filling load [18]. This might explain why reduced MAPSE, as a function of LV hemodynamic load, was revealed as the most sensitive marker of TS-LGAS in LGAS patients with LVEF > 50%. Additionally, reduction in TDI-s´, another parameter reflecting LV longitudinal dysfunction, is also suggestive of TS-LGAS in AS patients with LVEF ≥ 50%.
Echocardiographic determinants of TS-LGAS in patients with reduced LVEF
In LGAS patients with reduced LVEF, the disease features include both AS and heart failure. Ventricular remodeling (myocyte hypertrophy and myocardial fibrosis), systolic and diastolic dysfunction, in the setting of valvular stenosis and heart failure scenarios, contributes to the progressively elevated LV filling pressure, and/or left atrial pressure, which in turn would transmit to the pulmonary vasculature, inducing pulmonary venous congestion and pulmonary hypertension, so called WHO Group II - pulmonary hypertension [19, 20]. A previous study showed that pulmonary hypertension was presented in the majority of patients with severe aortic stenosis, and ejection fraction serves as one of the correlates of pulmonary hypertension in patients with severe aortic stenosis [21]. Another study indicated that both smaller aortic valve area and lower LVEF were responsible for the presence of pulmonary hypertension in a cohort of 626 patients with severe aortic stenosis [22]. In line with above findings, we found that increased systolic pulmonary pressure is an independent determinant of TS in LGAS patients with reduced LVEF. Another finding of the present study is that lower AV velocity ratio is related to the presence of true severe in LGAS patients with reduced LVEF. This parameter is only recommended in the EAE/ASE recommendations, a value of < 0.25 is suggestive of severe AS [23]. In our cohort, this value ranged from 0.23 at rest and 0.24 during DSE in TS-LGAS patients with LVEF > 50%, and 0.19 at rest and 0.19 during DSE in TS-LGAS patients with reduced LVEF. A previous study reported that velocity ratios could not only define the severity of AS, but also predict outcomes in patients with TS-LGAS and preserved LVEF [24]. Our results suggest AV velocity ratio could be used as an independent predictor for defining TS in LGAS patients with reduced LVEF.
Limitations
This retrospective study was performed monocentric and the number of patients included in the sub-cohorts was relatively small. The results of the present study might thus be affected by patient selection bias. Future studies with large patient cohort are warranted to validate the results obtained from this study. In this study, projected aortic valve area assessed by DSE was used to define the severity of AS [25]. Other imaging modalities may aid in the diagnosis of severe AS. Macroscopic evaluation of the valve at the time of valve replacement or measurement of aortic valve calcium score by multislice computed tomography could provide additional accuracy to differ TS- from PS-AS [26]. Due to the lack of available CT data for AV calcium score in our cohort, the severity of AS was only determined by projected aortic valve area. This study limitation should be considered in the interpretation of results derived from the current study.
Clinical implications
Our study results suggest that DSE enables evaluation of the severity of AS both in LGAS patients with reduced LVEF and in those with preserved LVEF, which is consistent with what has been demonstrated by previous studies [13, 26]. In the present study, we further explored the echocardiographic determinants suggestive of TS-LGAS. Reduced MAPSE and TDI-s´ are suggestive of TS in LGAS patients with preserved LVEF, while increased SPAP and lower AV velocity ratio are suggestive of TS in LGAS patients with reduced LVEF. These findings provide incremental information on the diagnosis and therapy decision for LGAS patients, particularly in those patients who cannot tolerate DSE due to the presence of contraindications. Patients with related echocardiographic features should be carefully evaluated for the AS severity with alternative complementary imaging modalities, such as measurement of aortic valve calcium score assessed by multislice computed tomography.
Conclusions
DSE is valuable for staging of LGAS in patients with both reduced and preserved LVEF. Low longitudinal LV function (MAPSE) and septal TDI-s´ are associated with TS-LGAS in patients with preserved LVEF, and high trans-tricuspid pressure gradient (SPAP) and low AV velocity ratio are associated with TS-LGAS in patients with reduced LVEF.
References
Bonow RO, Carabello BA, Kanu C, de Leon AC Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, O’Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Nishimura R, Page RL, Riegel B (2006) ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 114:e84–e231. https://doi.org/10.1161/CIRCULATIONAHA.106.176857
Clavel MA, Dumesnil JG, Capoulade R, Mathieu P, Senechal M, Pibarot P (2012) Outcome of patients with aortic stenosis, small valve area, and low-flow, low-gradient despite preserved left ventricular ejection fraction. J Am Coll Cardiol 60:1259–1267. https://doi.org/10.1016/j.jacc.2011.12.054
Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P (2007) Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 115:2856–2864. https://doi.org/10.1161/CIRCULATIONAHA.106.668681
Grayburn PA (2006) Assessment of low-gradient aortic stenosis with dobutamine. Circulation 113:604–606. https://doi.org/10.1161/CIRCULATIONAHA.105.600684
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, Thomas JD, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Creager MA, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Stevenson WG, Yancy CW (2014) 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg 148:e1–e132. https://doi.org/10.1016/j.jtcvs.2014.05.014
Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, Douglas PS, Faxon DP, Gillam LD, Kimball TR, Kussmaul WG, Pearlman AS, Philbrick JT, Rakowski H, Thys DM, Antman EM, Smith SC Jr, Alpert JS, Gregoratos G, Anderson JL, Hiratzka LF, Hunt SA, Fuster V, Jacobs AK, Gibbons RJ, Russell RO (2003) ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation 108:1146–1162. https://doi.org/10.1161/01.CIR.0000073597.57414.A9
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28:1–39 e14. https://doi.org/10.1016/j.echo.2014.10.003
Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22:107–133. https://doi.org/10.1016/j.echo.2008.11.023
Chahal NS, Lim TK, Jain P, Chambers JC, Kooner JS, Senior R (2010) Normative reference values for the tissue Doppler imaging parameters of left ventricular function: a population-based study. Eur J Echocardiogr 11:51–56. https://doi.org/10.1093/ejechocard/jep164
Liu D, Hu K, Stork S, Herrmann S, Kramer B, Cikes M, Gaudron PD, Knop S, Ertl G, Bijnens B, Weidemann F (2014) Predictive value of assessing diastolic strain rate on survival in cardiac amyloidosis patients with preserved ejection fraction. PloS ONE 9:e115910. https://doi.org/10.1371/journal.pone.0115910
Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M (2009) Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr 10:1–25. https://doi.org/10.1093/ejechocard/jen303
Nishimura RA, Grantham JA, Connolly HM, Schaff HV, Higano ST, Holmes DR Jr (2002) Low-output, low-gradient aortic stenosis in patients with depressed left ventricular systolic function: the clinical utility of the dobutamine challenge in the catheterization laboratory. Circulation 106:809–813
Clavel MA, Burwash IG, Mundigler G, Dumesnil JG, Baumgartner H, Bergler-Klein J, Senechal M, Mathieu P, Couture C, Beanlands R, Pibarot P (2010) Validation of conventional and simplified methods to calculate projected valve area at normal flow rate in patients with low flow, low gradient aortic stenosis: the multicenter TOPAS (True or Pseudo Severe Aortic Stenosis) study. J Am Soc Echocardiogr 23:380–386. https://doi.org/10.1016/j.echo.2010.02.002
Clavel MA, Ennezat PV, Marechaux S, Dumesnil JG, Capoulade R, Hachicha Z, Mathieu P, Bellouin A, Bergeron S, Meimoun P, Arsenault M, Le Tourneau T, Pasquet A, Couture C, Pibarot P (2013) Stress echocardiography to assess stenosis severity and predict outcome in patients With paradoxical low-flow, low-gradient aortic stenosis and preserved LVEF. Jacc Cardiovasc Imag 6:175–183. https://doi.org/10.1016/j.jcmg.2012.10.015
Herrmann S, Stork S, Niemann M, Lange V, Strotmann JM, Frantz S, Beer M, Gattenlohner S, Voelker W, Ertl G, Weidemann F (2011) Low-gradient aortic valve stenosis myocardial fibrosis and its influence on function and outcome. J Am Coll Cardiol 58:402–412. https://doi.org/10.1016/j.jacc.2011.02.059
Rydberg E, Gudmundsson P, Kennedy L, Erhardt L, Willenheimer R (2004) Left atrioventricular plane displacement but not left ventricular ejection fraction is influenced by the degree of aortic stenosis. Heart 90:1151–1155. https://doi.org/10.1136/hrt.2003.020628
Matos JD, Kiss JE, Locke AH, Goldberg RI, Mukamal K, Manning WJ (2017) Relation of the mitral annular plane systolic excursion to risk for intervention in initially asymptomatic patients with aortic stenosis and preserved systolic function. Am J Cardiol 120:2031–2034. https://doi.org/10.1016/j.amjcard.2017.08.021
Ross J Jr (1985) Afterload mismatch in aortic and mitral valve disease: implications for surgical therapy. J Am Coll Cardiol 5:811–826
Fang JC, DeMarco T, Givertz MM, Borlaug BA, Lewis GD, Rame JE, Gomberg-Maitland M, Murali S, Frantz RP, McGlothlin D, Horn EM, Benza RL (2012) World Health Organization pulmonary hypertension group 2: pulmonary hypertension due to left heart disease in the adult–a summary statement from the pulmonary hypertension Council of the International Society for heart and lung transplantation. J Heart Lung Transplant 31:913–933. https://doi.org/10.1016/j.healun.2012.06.002
Pellegrini P, Rossi A, Pasotti M, Raineri C, Cicoira M, Bonapace S, Dini FL, Temporelli PL, Vassanelli C, Vanderpool R, Naeije R, Ghio S (2014) Prognostic relevance of pulmonary arterial compliance in patients with chronic heart failure. Chest 145:1064–1070. https://doi.org/10.1378/chest.13-1510
Ben-Dor I, Goldstein SA, Pichard AD, Satler LF, Maluenda G, Li Y, Syed AI, Gonzalez MA, Gaglia MA Jr, Wakabayashi K, Delhaye C, Belle L, Wang Z, Collins SD, Torguson R, Okubagzi P, Aderotoye A, Xue Z, Suddath WO, Kent KM, Epstein SE, Lindsay J, Waksman R (2011) Clinical profile, prognostic implication, and response to treatment of pulmonary hypertension in patients with severe aortic stenosis. Am J Cardiol 107:1046–1051. https://doi.org/10.1016/j.amjcard.2010.11.031
Kapoor N, Varadarajan P, Pai RG (2008) Echocardiographic predictors of pulmonary hypertension in patients with severe aortic stenosis. Eur J Echocardiogr 9:31–33. https://doi.org/10.1016/j.euje.2007.01.005
Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M (2009) Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 22:1–23. https://doi.org/10.1016/j.echo.2008.11.029 quiz 101–102.
Jander N, Hochholzer W, Kaufmann BA, Bahlmann E, Gerdts E, Boman K, Chambers JB, Nienaber CA, Ray S, Rossebo A, Pedersen TR, Wachtell K, Gohlke-Barwolf C, Neumann FJ, Minners J (2014) Velocity ratio predicts outcomes in patients with low gradient severe aortic stenosis and preserved EF. Heart 100:1946–1953. https://doi.org/10.1136/heartjnl-2014-305763
Annabi MS, Touboul E, Dahou A, Burwash IG, Bergler-Klein J, Enriquez-Sarano M, Orwat S, Baumgartner H, Mascherbauer J, Mundigler G, Cavalcante JL, Larose E, Pibarot P, Clavel MA (2018) Dobutamine stress echocardiography for management of low-flow, low-gradient aortic stenosis. J Am Coll Cardiol 71:475–485. https://doi.org/10.1016/j.jacc.2017.11.052
Clavel MA, Ennezat PV, Marechaux S, Dumesnil JG, Capoulade R, Hachicha Z, Mathieu P, Bellouin A, Bergeron S, Meimoun P, Arsenault M, Le Tourneau T, Pasquet A, Couture C, Pibarot P (2013) Stress echocardiography to assess stenosis severity and predict outcome in patients with paradoxical low-flow, low-gradient aortic stenosis and preserved LVEF. JACC Cardiovasc Imaging 6:175–183. https://doi.org/10.1016/j.jcmg.2012.10.015
Acknowledgements
This work was supported by grants from the Bundesministerium für Bildung und Forschung of the Federal Republic of Germany (BMBF 01EO1504 MO.6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest.
Ethical approval
The study was conducted in accordance to the Declaration of Helsinki and approved by the Local Ethics Committee at the University of Würzburg (AZ 11/03 and 60/14). Informed consent was obtained from all patients or their guardians.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Liu, D., Hu, K., Liebner, E. et al. Value of low-dose dobutamine stress echocardiography on defining true severe low gradient aortic stenosis in patients with preserved left ventricular ejection fraction. Int J Cardiovasc Imaging 34, 1877–1887 (2018). https://doi.org/10.1007/s10554-018-1416-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-018-1416-z