Abstract
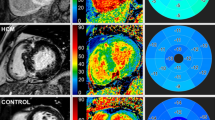
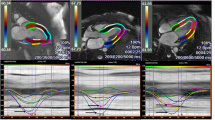
Cardiac amyloidosis (CA) is a significant contributor to heart failure with preserved ejection fraction and is appreciating expanding therapeutic options. Non-invasive tools aimed at accurate identification and surveillance of therapeutic response are of immediate and expanding need. While native and post-contrast T1 mapping quantify expansion of the extra-cellular compartment from amyloid protein deposition, 3D strain analysis of non-contrast cine images offers unique advantages relevant to high prevalence of renal insufficiency in this population and reduced dependency on field strength, pulse sequence, and vendor implementation. We aimed to evaluate global and segmental associations between 3D strain and T1 mapping in patients with cardiac amyloidosis. Twenty consecutive patients with confirmed CA were recruited and underwent a standardized cardiovascular magnetic resonance imaging protocol at 3 T including using multi-planar cine imaging and T1 mapping using a shortened modified look-locker inversion recovery sequence. T1 mapping was performed pre- and (when permitted by renal function) post-contrast and measured for segmental T1 values. Spatially-matched 3D strain-based measures were similarly calculated. Mean left ventricular ejection fraction was 61 ± 21% (range 30–73%). Mean global native T1 was 1308 ± 96 ms. Post-contrast T1 and partition coefficient were 558 ± 104 ms and 0.85 ± 0.31, respectively. Global myocardial strain values were 8.1 ± 2.9% in the longitudinal direction, − 9.2 ± 3.4% in the circumferential direction, and 41.7 ± 22.8% in the maximum principal direction. Segmental analyses confirmed relative worsening in T1 values and reductions in strain values in the basal myocardial segments with relative sparing of the apical segments. Significant associations between T1 and strain-based measures were observed globally and segmentally, with the strongest associations found both globally and segmentally in the circumferential and minimum principal directions of deformation. This study identifies strong associations between 3D myocardial strain and T1-mapping based markers of regional amyloid protein deposition. These findings support expanded investigation of myocardial strain as a surrogate marker of response to novel therapeutic strategies in patients with cardiac amyloidosis.




Similar content being viewed by others
Abbreviations
- CA:
-
Cardiac amyloidosis
- ACE:
-
Angiotensin converting enzyme
- ARB:
-
Angiotensin II receptor blocker
- AL:
-
Amyloid light chain
- ATTR:
-
Amyloid transthyretin
- COPD:
-
Chronic obstructive pulmonary disease
- CMR:
-
Cardiovascular magnetic resonance imaging
- HFpEF:
-
Heart failure with preserved ejection fraction
- LV:
-
Left ventricular
- RV:
-
Right ventricular
- LA:
-
Left atrial
- LVEF:
-
Left ventricular ejection fraction
- RVEF:
-
Right ventricular ejection fraction
- ShMOLLI:
-
Shortened modified look-locker inversion recovery
- LGE:
-
Late gadolinium enhancement
- QOL:
-
Quality of life
- RRSR:
-
Relative regional strain ratio
References
Alexander KM, Singh A, Falk RH (2017) Novel pharmacotherapies for cardiac amyloidosis. Pharmacol Ther 180: 129–138. https://doi.org/10.1016/j.pharmthera.2017.06.011
Gertz MA, Landau H, Comenzo RL, Seldin D, Weiss B, Zonder J, Merlini G, Schonland S, Walling J, Kinney GG, Koller M, Schenk DB, Guthrie SD, Liedtke M (2016) First-in-human phase I/II study of NEOD001 in patients with light chain amyloidosis and persistent organ dysfunction. J Clin Oncol 34(10):1097–1103. https://doi.org/10.1200/JCO.2015.63.6530
Hanna M (2014) Novel drugs targeting transthyretin amyloidosis. Curr Heart Fail Rep 11(1):50–57. https://doi.org/10.1007/s11897-013-0182-4
Castano A, Helmke S, Alvarez J, Delisle S, Maurer MS (2012) Diflunisal for ATTR cardiac amyloidosis. Congest Heart Fail 18(6):315–319. https://doi.org/10.1111/j.1751-7133.2012.00303.x
Sekijima Y, Tojo K, Morita H, Koyama J, Ikeda S (2015) Safety and efficacy of long-term diflunisal administration in hereditary transthyretin (ATTR) amyloidosis. Amyloid 22(2):79–83. https://doi.org/10.3109/13506129.2014.997872
Higaki JN, Chakrabartty A, Galant NJ, Hadley KC, Hammerson B, Nijjar T, Torres R, Tapia JR, Salmans J, Barbour R, Tam SJ, Flanagan K, Zago W, Kinney GG (2016) Novel conformation-specific monoclonal antibodies against amyloidogenic forms of transthyretin. Amyloid 23(2):86–97. https://doi.org/10.3109/13506129.2016.1148025
Castano A, Manson DK, Maurer MS, Bokhari S (2017) Transthyretin cardiac amyloidosis in older adults: optimizing cardiac imaging to the corresponding diagnostic and management goal. Curr Cardiovasc Risk Rep 11(https://doi.org/10.1007/s12170-017-0541-x
Hutt DF, Quigley A-M, Page J, Hall ML, Burniston M, Gopaul D, Lane T, Whelan CJ, Lachmann HJ, Gillmore JD, Hawkins PN, Wechalekar AD (2014) Utility and limitations of 3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in systemic amyloidosis. Eur Heart J 15(11):1289–1298. https://doi.org/10.1093/ehjci/jeu107
Karamitsos TD, Neubauer S (2015) T1 mapping and amyloid cardiomyopathy: how much better can it get? Eur Heart J 36(4):203–205. https://doi.org/10.1093/eurheartj/ehu442
Ruberg FL, Appelbaum E, Davidoff R, Ozonoff A, Kissinger KV, Harrigan C, Skinner M, Manning WJ (2009) Diagnostic and prognostic utility of cardiovascular magnetic resonance imaging in light-chain cardiac amyloidosis. Am J Cardiol 103(4):544–549. https://doi.org/10.1016/j.amjcard.2008.09.105
Khawaja AZ, Cassidy DB, Al Shakarchi J, McGrogan DG, Inston NG, Jones RG (2015) Revisiting the risks of MRI with Gadolinium based contrast agents-review of literature and guidelines. Insights Imaging 6(5):553–558
Williams LK, Forero JF, Popovic ZB, Phelan D, Delgado D, Rakowski H, Wintersperger BJ, Thavendiranathan P (2017) Patterns of CMR measured longitudinal strain and its association with late gadolinium enhancement in patients with cardiac amyloidosis and its mimics. J Cardiovasc Magn Reson 19(1):61. https://doi.org/10.1186/s12968-017-0376-0
Schuster A, Paul M, Bettencourt N, Morton G, Chiribiri A, Ishida M, Hussain S, Jogiya R, Kutty S, Bigalke B, Perera D, Nagel E (2013) Cardiovascular magnetic resonance myocardial feature tracking for quantitative viability assessment in ischemic cardiomyopathy. Int J Cardiol 166(2):413–420
Piras P, Teresi L, Puddu PE, Torromeo C, Young AA, Suinesiaputra A, Medrano-Gracia P (2017) Morphologically normalized left ventricular motion indicators from MRI feature tracking characterize myocardial infarction. Sci Rep 7(1):12259
Lo Q, Haluska B, Chia EM, Lin MW, Richards D, Marwick T, Thomas L (2016) Alterations in regional myocardial deformation assessed by strain imaging in cardiac amyloidosis. Echocardiography 33(12):1844–1853. https://doi.org/10.1111/echo.13355
Karamitsos TD, Piechnik SK, Banypersad SM, Fontana M, Ntusi NB, Ferreira VM, Whelan CJ, Myerson SG, Robson MD, Hawkins PN, Neubauer S, Moon JC (2013) Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging 6(4):488–497. https://doi.org/10.1016/j.jcmg.2012.11.013
White JA, Kim HW, Shah D, Fine N, Kim KY, Wendell DC, Al-Jaroudi W, Parker M, Patel M, Gwadry-Sridhar F, Judd RM, Kim RJ (2014) CMR imaging with rapid visual T1 assessment predicts mortality in patients suspected of cardiac amyloidosis. JACC Cardiovasc Imaging 7(2):143–156. https://doi.org/10.1016/j.jcmg.2013.09.019
Bokhari S, Shahzad R, Castano A, Maurer MS (2014) Nuclear imaging modalities for cardiac amyloidosis. J Nucl Cardiol 21(1):175–184. https://doi.org/10.1007/s12350-013-9803-2
Piechnik SKFV, Dall’Armellina E, Cochlin LE, Greiser A, Neubauer S, Robson MD (2010) Shortened modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson 12: 69
Suinesiaputra A, Bluemke DA, Cowan BR, Friedrich MG, Kramer CM, Kwong R, Plein S, Schulz-Menger J, Westenberg JJ, Young AA, Nagel E (2015) Quantification of LV function and mass by cardiovascular magnetic resonance: multi-center variability and consensus contours. J Cardiovasc Magn Reson. https://doi.org/10.1186/s12968-015-0170-9
Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff-Brenkenhoff F, Kramer CM, Pennell DJ, Plein S, Nagel E (2013) Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson 15:35
Salerno M, Janardhanan R, Jiji RS, Brooks J, Adenaw N, Mehta B, Yang Y, Antkowiak P, Kramer CM, Epstein FH (2013) Comparison of methods for determining the partition coefficient of gadolinium in the myocardium using T1 mapping. J Magn Reson Imaging 38(1):217–224. https://doi.org/10.1002/jmri.23875
Diesbourg LD, Prato FS, Wisenberg G, Drost DJ, Marshall TP, Carroll SE, O’Neill B (1992) Quantification of myocardial blood flow and extracellular volumes using a bolus injection of Gd-DTPA: kinetic modeling in canine ischemic disease. Magn Reson Med 23(2):239–253
Satriano A, Heydari B, Narous M, Exner DV, Mikami Y, Attwood MM, Tyberg JV, Lydell CP, Howarth AG, Fine NM, White JA (2017) Clinical feasibility and validation of 3D principal strain analysis from cine MRI: comparison to 2D strain by MRI and 3D speckle tracking echocardiography. Int J Cardiovasc Imaging 33(12):1979–1992
Cohen A, Comenzo RL (2010) Systemic light-chain amyloidosis: advances in diagnosis, prognosis, and therapy. Hematol Am Soc Hematol Educ Progr 2010: 287–294
Vranian MN, Sperry BW, Valent J, Hanna M (2015) Emerging advances in the management of cardiac amyloidosis. Curr Cardiol Rep 17(11):100. https://doi.org/10.1007/s11886-015-0653-1
Narotsky DL, Castano A, Weinsaft JW, Bokhari S, Maurer MS (2016) Wild-type transthyretin cardiac amyloidosis: novel insights from advanced imaging. Can J Cardiol 32(9):1166e1–1166e10. https://doi.org/10.1016/j.cjca.2016.05.008
Banypersad SM, Moon JC, Whelan C, Hawkins PN, Wechalekar AD (2012) Updates in cardiac amyloidosis: a review. J Am Heart Assoc 1(2):e000364. https://doi.org/10.1161/JAHA.111.000364
Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P, Mascherbauer J, Nezafat R, Salerno M, Schelbert EB, Taylor AJ, Thompson R, Ugander M, van Heeswijk RB, Friedrich MG (2017) Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 19(1):75
Banypersad SM, Fontana M, Maestrini V, Sado DM, Captur G, Petrie A, Piechnik SK, Whelan CJ, Herrey AS, Gillmore JD, Lachmann HJ, Wechalekar AD, Hawkins PN, Moon JC (2015) T1 mapping and survival in systemic light-chain amyloidosis. Eur Heart J 36(4):244–251. https://doi.org/10.1093/eurheartj/ehu444
Maurer MS, Elliott P, Comenzo R, Semigran M, Rapezzi C (2017) Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation 135(14):1357–1377. https://doi.org/10.1161/CIRCULATIONAHA.116.024438
Perugini E, Rapezzi C, Piva T, Leone O, Bacchi-Reggiani L, Riva L, Salvi F, Lovato L, Branzi A, Fattori R (2006) Non-invasive evaluation of the myocardial substrate of cardiac amyloidosis by gadolinium cardiac magnetic resonance. Heart 92(3):343–349. https://doi.org/10.1136/hrt.2005.061911
Acknowledgements
Dr. White received salary support from the Heart and Stroke Foundation of Alberta during this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ms. Na’ama Avitzur declares that she has no conflict of interest. Dr. Alessandro Satriano declares that he has no conflict of interest. Dr. Muhammad Sarim Afzal declares that he has no conflict of interest. Ms. Mariam Narous declares that she has no conflict of interest. Dr. Yoko Mikami declares that she has no conflict of interest. Mr. Reis Hansen declares that he has no conflict of interest. Mr. Gary Dobko declares that he has no conflict of interest. Mrs. Jacqueline Flewitt declares that she has no conflict of interest. Dr. Carmen P. Lydell declares that she has no conflict of interest. Dr. Andrew G. Howarth receives consulting fees from Amgen. Dr. Kelvin Chow is employed by Siemens Medical Solutions USA. Inc. Dr. Nowell M. Fine receives consulting fees from Novartis and Pfizer. Dr. James A. White received salary support from the Heart and Stroke Foundation of Alberta during this study, receives research grants from Circle Cardiovascular Inc., and is a shareholder of Cohesic Inc.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Avitzur, N., Satriano, A., Afzal, M. et al. 3D myocardial deformation analysis from cine MRI as a marker of amyloid protein burden in cardiac amyloidosis: validation versus T1 mapping. Int J Cardiovasc Imaging 34, 1937–1946 (2018). https://doi.org/10.1007/s10554-018-1410-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-018-1410-5