Abstract
The aim of this study was to compare neointima proliferation in three drug-eluting stents (DES) produced by the same company (Balton, Poland) which are covered with a biodegradable polymer and elute sirolimus (concentration: 1.0 and 1.2 µg/mm2), but have different stent platforms and strut thickness: stainless steel Prolim® (115 µm) and BiOSS LIM® (120 µm) and cobalt-chromium Alex® (70 µm). We analyzed data of patients with quantitative coronary angiography (QCA) and optical coherence tomography (OCT) at 12 months from BiOSS LIM Registry, Prolim Registry and Alex OCT clinical trial. There were 56 patients enrolled, in whom 29 Prolim® stents were deployed, in 11—BiOSS LIM® and in 16—Alex stents. The late lumen loss was the smallest in Prolim® subgroup (0.26 ± 0.17 mm) and did not differ from Alex® subgroup (0.28 ± 0.47 mm). This parameter was significantly bigger in BiOSS® subgroup (0.38 ± 0.19 mm; p < 0.05). In OCT analysis there was no statistically significant difference between Prolim® and Alex® subgroups in terms of mean neointima burden (24.6 ± 8.6 vs. 19.27 ± 8.11%) and neointima volume (28.16 ± 15.10 vs. 24.51 ± 17.64 mm3). In BiOSS® group mean neointima burden (30.9 ± 6.2%) and mean neointima volume (44.9 ± 4.9 mm3) were significantly larger. The morphological analysis revealed that in most cases in all groups the neointima was homogenous with plaque presence only around stent struts. In the QCA and OCT analysis regular DES (Prolim® and Alex®) obtained similar results, whereas more pronounced response from the vessel wall was found in the BiOSS® subgroup.
Similar content being viewed by others
Introduction
Drug eluting stents (DES) reduce the incidence of restenosis and thereby also the incidence of repeated revascularizations. Initially, most stents were made of stainless steel and therefore had relatively thick struts. Today the world applies the platinum-chromium or cobalt-chromium alloys as the stent platform, what makes possible to produce much thinner struts (even half smaller). The ISAR-STEREO trial demonstrated that a thin-strut stent (≤ 100 µm) had a lower rate of restenosis than a thick strut stent (> 100 µm) of similar design [1]. Comparable results were found in several other trials [2,3,4]. The abovementioned studies suggested that thick struts might predispose to excessive neointima proliferation, however, these results are based on studies with bare metal stents or the first generation DES presently not available on the market.
The deployment of a durable polymer DES (DP-DES) is a standard of care in patients with coronary artery lesions. However, studies assessing biodegradable polymer DES (BP-DES) proved the non-inferiority to DP-DES with the expectation for the decreased inflammatory response after stent implantation and, in consequence, for faster vessel healing [5].
The aim of this study was to compare neointima proliferation in three DES produced by the same company (Balton, Poland) which are covered with the same biodegradable polymer and elute the same drug (sirolimus concentrations: 1.0 µg/mm2 for Alex® and BiOSS LIM®, while 1.2 µg/mm2 Prolim®) but have different stent platforms: stainless steel (Prolim®, BiOSS LIM®) or cobalt-chromium (Alex®) and strut thickness: 115, 120 and 70 µm, respectively.
Materials and methods
Study population and study design
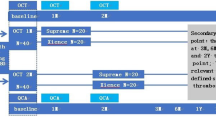
We included patients who had implanted one of the following stents (Prolim®, Alex® or BiOSS LIM®) and had performed optical coherence tomography (OCT) during 12-month angiographic follow-up. Patients participated in one of the following studies: Prolim Registry, BiOSS LIM Registry or Alex OCT study. The detailed inclusion and exclusion criteria are described elsewhere [6,7,8]. The appropriate Ethics Committees approved study protocols.
Study device
The BiOSS LIM® is a coronary, dedicated bifurcation balloon-expandable stent made of 316L stainless steel (strut thickness 120 μm; strut width 180 μm). The cover ratio is 18%. The stent consists of two parts, proximal and distal, joined with two connecting struts (depending on stent size: 1.8–2.3 mm in length) at the step-up middle zone. The proximal part of the stent has a larger diameter in relation to the distal part (diameter ratio of proximal to distal parts is included between 1.15 and 1.3). Maximal diameter of expanded stent cell is 3.5 mm [8].
The Prolim® stent is a balloon expandable coronary stent with RX delivery system. The stent platform is made of a laser-cut 316L metallic tube with a wall thickness of 115 µm and strut width of 80 µm. The cover ratio is 19%. Maximal diameter of expanded stent cell is 1.8 mm [9].
The Alex® stent is a balloon expandable coronary stent with RX delivery system. The stent platform is made of a laser-cut cobalt-chromium tube with a wall thickness of 70 µm and strut width of 75 µm. The cover ratio is 18%. Maximal diameter of expanded stent cell is 1.5 mm [9].
All abovementioned stents are covered with a mixture of biodegradable poly(lactide-co-glycolide) copolymer and an antiproliferative substance—sirolimus. The polymer layers release sirolimus (1.0 µg/mm2 for Alex and BiOSS LIM and 1.2 µg/mm2 for Prolim) in a time-controlled process due to their biodegradation (lasting around 8 weeks) [10].
Procedure
Percutaneous coronary interventions (PCI) were performed according to local standards via radial or femoral access using 6 Fr or 7 Fr guiding catheters. Pharmacological treatment was according to the most recent guidelines [11]. Troponin I (TnI), creatine kinase (CK) and creatine kinase-myocardial band (CK-MB) were measured pre-procedural, 6 and 24 h after procedure in all patients. Periprocedural myocardial infarction (type 4a) was defined according to the third universal definition [12].
Follow-up
The assessment of the anginal status, data collection of adverse events, details of any subsequent coronary interventions, and the use and changes in concomitant medications were collected at 12 ± 0.5 months. The angiographic control was planned at 12 months, in which patients in Prolim and BiOSS LIM Registries had OCT examination randomly (approximately 15% of patients), whereas in the Alex OCT study it was mandatory at 12 months.
Endpoints
The primary endpoint was the cumulative rate of major adverse cardiovascular events (MACE) consisting of cardiac death, myocardial infarction (MI) and clinically-driven target lesion revascularization (TLR). Secondary endpoints included cardiac death, all-cause death, MI, TLR, stent thrombosis, late lumen loss (LLL) assessed in quantitative coronary angiography (QCA), the percentage of covered struts and neointima volume and morphology characteristics assessed in OCT as well as the device success rate. Cardiac death included death resulting from an acute MI, sudden cardiac death, death due to heart failure and death due to cardiac procedures. All deaths were deemed cardiac unless proven otherwise. MI was defined according to third universal definition [12]. Clinically-driven TLR was defined as reintervention of the target lesion due to presence of a symptomatic ≥ 50% diameter stenosis during follow-up. Device success was defined as successful deployment of the intended stent in the target site without a system failure. The definite stent thrombosis was defined as state with symptoms suggestive of an acute coronary syndrome and angiographic or pathologic confirmation of stent thrombosis. The probable stent thrombosis was defined as the unexplained death within 30 days or target vessel myocardial infarction without angiographic confirmation of stent thrombosis, and the possible stent thrombosis was defined as any unexplained death after 30 days [13].
Quantitative angiography analysis
All coronary angiograms were recorded after intracoronary administration of 200 μg of nitroglycerin. Two orthogonal views were chosen to visualize the target lesion. A QCA analysis was performed using commercially available software (QCA-CMS version 5.0, Medis, Leiden, the Netherlands). Catheter calibration was used in all cases. The following parameters: lesion length, reference vessel diameter, minimal lumen diameter, % diameter stenosis, acute lumen gain and LLL were calculated as described previously [14].
Optical coherent tomography analysis
Briefly, after wiring the artery with the guidewire as described previously, the Dragon Fly catheter (LigthLab Co.) was advanced distally to the implanted stent and during continuous contrast media flush (Iodixanol, Visipaque GE Healthcare), the automatic pullback was performed. The commercially available console (M2 or M3 by LigthLab Co.) was used. Optical coherence tomography images were obtained along the region of interest, which was the implanted stent plus 5 mm both proximal and distal. Off-line analysis was performed after careful recalibration of acquired images along the reconstructed longitudinal segment. Calibration was obtained by adjusting the z-offset, the zero-point setting of the system. The analysis was performed applying a dedicated off-line software (St Jude Medical). Quantitative measurements of the minimal lumen area and minimal lumen diameter were obtained in all consecutive frames along the region of interest using semi-automated algorithm. Additionally, the mean value of all lumen area cross-sections measured inside the region was calculated. Additionally, lumen volume analysis was performed along region of interest—all measured lumen area cross-sections were summed. Mean neointimal burden was calculated as the ratio of the mean neointima area to the mean stent area [7, 15].
Moreover, to assess stent apposition OCT analysis was performed every 0.2 mm of the stent. The stent struts apposition was classified as: (1) apposed (2) protruded and (3) malapposed according to a distance length between vessel wall and center of the stent strut. If such distance was: (1) more than 130 μm, malapposition was detected, (2) in range of 20 to 130 μm, protrusion was detected. The morphology of the neointima was analyzed according to the previously validated OCT criteria, and classified as type I (thin cap neoatheroma, lipid-rich), type II (thick-cap, layered), type III (peri-strut, homogenous) and type IV (pre-existing, homogenous) [16, 17].
Statistical analysis
Continuous variables were presented as mean ± standard deviation. Categorical data were presented as numbers (%). Continuous variables were compared using an ANOVA test, and categorical data using the χ2 test. If distribution was not normal (verified with the Shapiro–Wilk test) for continuous variables Kruskal–Wallis test was used. P values of < 0.05 were considered statistically significant. If P was < 0.05 for determining the statistical significance between groups appropriate post-hoc tests were used. Pearson correlation was applied in continuous variables. Additionally, univariate and multivariate linear regression analyses were performed. Statistical analyses were performed using R 3.0.2 for OS (R Foundation, Vienna, Austria).
Results
Baseline clinical and angiographic characteristics
A total of 56 patients were enrolled into this analysis, i.e. 11 patients—with BiOSS LIM® stent implanted, 29 patients with Prolim®, and 16 patients—with Alex® stent deployed. The baseline characteristics was comparable between groups, apart from the age. The mean age was significantly higher in the Prolim® subgroup than in the BiOSS® subgroup (68 ± 10 vs. 60 ± 6 years, p < 0.05). The mean age in Alex group was 62 ± 9 years. The detailed clinical characteristics is presented in Table 1.
In the Prolim® and BiOSS LIM® subgroups most patients presented with multivessel disease and in all groups lesions were of the moderate complexity. In the Prolim® and BiOSS® subgroups lesions were located most frequently in left anterior descending artery, 48.3 and 72.7%, respectively. In the Alex® subgroup the left circumflex artery was the most frequently stented vessel (43.8%). More details are presented in Table 2.
Procedural characteristics
The main procedural variables are presented in Table 3. The device success rate was 100% in all subgroups. There were no significant differences in procedural details as well as in the rate of periprocedural complications in those three groups.
Clinical outcomes
The clinical follow-up at 12 months was available in all patients. The MACE rate was: 9.1, 0 and 6.25% in the BiOSS LIM®, Prolim® and Alex® subgroups, respectively. In the observation there was one case of TLR treated with another DES in the BiOSS® subgroup as well as in the Alex® subgroup. There was no death or stent thrombosis.
Quantitative coronary angiography and optical coherence tomography analysis
The QCA data are presented in Table 4. The immediate angiographic success rate was 100%. Acute lumen gain was the lowest in the BiOSS® subgroup (1.35 ± 0.23 mm) and significantly differed from acute lumen gain in the Prolim® group (1.86 ± 0.39 mm) as well as in the Alex® subgroup (1.78 ± 0.47 mm). Whereas the late lumen loss was the smallest in the Prolim® subgroup (0.26 ± 0.17 mm) and it was significantly lower than in the BiOSS® subgroup (0.38 ± 0.19 mm). The LLL in the Alex® subgroup was 0.28 ± 0.47 mm, but due to relatively high standard deviation (among others due to one case of TLR) it did not differ significantly between the other two subgroups (Fig. 1a). Worth mentioning is the fact that when analyzing the BiOSS LIM® stent as two parts with different diameter (3.57 ± 0.12 × 3.0 ± 0.05 mm) we obtained the LLL in the proximal part of 0.36 ± 0.25 mm, and in the distal part of 0.39 ± 0.13 mm (p = NS).
The OCT analysis data are presented in Table 5. The OCT at 12 months was performed in all patients. The rate of embedded stent struts was comparable between Prolim® and Alex® subgroups (98.6 and 99.2%), and was significantly lower in the BiOSS® subgroup (95.8%). Similarly, there was no statistically significant difference between Prolim® and Alex® subgroups in terms of mean neointima burden (24.6 ± 8.6 vs. 19.27 ± 8.11%) and neointima volume (28.16 ± 15.10 vs. 24.51 ± 17.64 mm3), while in BiOSS® group these parameters were significantly larger, 30.9 ± 6.2% and 44.9 ± 4.9 mm3 (Fig. 1b–d). Moreover, as shown on the Fig. 2 in each group LLL values significantly correlated with OCT parameters: neointima area, neointima volume and neointima burden.
The morphological analysis revealed that in most cases in all groups the neointima was homogenous with plaque presence only around the stent struts. Patterns did not differ significantly (Table 5).
In Tables 6 and 7 there are presented linear regression analyses for LLL value and for neointima burden value, respectively. Regarding LLL stent length was the only significantly correlating value both in univariate (regression coefficient − 0.021, 95% CI − 0.040 to − 0.002, p = 0.03) as well as in multivariate analysis (− 0.025, 95% CI − 0.046 to − 0.004, p = 0.02). Whereas when neointima burden was analyzed the following factors were significant in the univariate analysis: strut width (87.676, 95% CI 30.305–145.048, p = 0.003) and strut thickness (151.185, 95% CI 38.862–263.508, p = 0.009) as well as in the multivariate analysis: strut width (66.406, 95% CI − 1.957 to 134.768, p = 0.04) and postdilatation (− 4.860, 95% CI − 9.982 to 0.263, p = 0.04).
The new cut-off of real thin-strut stents
When analyzing the abovementioned parameters we decided to verify the hypothesis that not only strut thickness as one strut dimension is responsible for vascular response, but 2-dimensional parameter is more accurate (Figs. 3, 4). Therefore, we have introduced a new parameter (strut cross-sectional area—StrCSA) that is the product of strut width and its thickness. The following values were obtained: for Alex®—0.005250 mm2, for Prolim®—0.009200 mm2, and for BiOSS LIM®—0.021600 mm2.
Discussion
The LLL is a parameter widely used for assessment of the stent’s performance. We found that it was comparable in the Alex® and Prolim® subgroups (0.28 ± 0.47 and 0.26 ± 0.17 mm, respectively), whereas it was significantly bigger in the BiOSS® subgroup (0.38 ± 0.19 mm, p < 0.05). It is worth stressing that there was no difference between LLL values for proximal and distal part of BiOSS® LIM stent. The lack of differences between Alex® and Prolim® may be at least partly explained by higher sirolimus concentration on this second stent (1.2 vs. 1.0 µg/mm2). If we take into account the same biodegradable polymer one can say that sirolimus in higher concentration reduced the effect of thicker struts of Prolim® stent.
The obtained results were better than those observed in Cypher® stent (0.40 ± 0.65 mm, strut thickness 140 µm) [18], but worse than in the newest generation stents: for Excel II® stent − 0.12 ± 0.34 mm (strut thickness 88 µm), for Orsiro® stent—0.10 ± 0.32 mm (strut thickness 60 µm), for Xience® stent—0.11 ± 0.29 mm (strut thickness 80 µm), and for Supralimus® stent—0.09 ± 0.37 mm (strut thickness 66 µm) [19,20,21]. It should be emphasized that the highest sirolimus concentration was for Cypher® comparing with Orsiro® and Supralimus® stents (1.4, 1.2 and 1.2 µg/mm2, respectively).
In our study regarding LLL stent length was the only significantly correlating value both in univariate (regression coefficient − 0.021, 95% CI − 0.040 to − 0.002, p = 0.03) as well as in multivariate analysis (− 0.025, 95% CI − 0.046 to − 0.004, p = 0.02).
There are many factors influencing on the vessel wall response to stent implantation. There is not only strut thickness and the drug’s type but also the type of stent platform and drug’s carrier, drug itself (including concentration) as well as accompanying diseases (such as diabetes mellitus). Strut width is rather forgotten and generally not analyzed parameter, because in most contemporary stents the cross-sectional area of the strut has the shape of the circle or square (width = thickness). This is a mistake in our opinion. After all, strut width determines the area of stent adhering to the wall that initiates vascular response, while strut thickness is probably responsible for the duration of neointima proliferation. Therefore, it is rationale that for standard vascular response assessment one should use the “product” of these two parameters (e.g. StrCSA) as better illustrating the geometric form of the stent strut and its potential impact on neointimal proliferation magnitude.
The analysis of the proposed parameter for all analyzed stents entitled to presume that its relatively low LLL value for Prolim® stent was associated with the smaller value of strut width compared with BiOSS LIM® (Alex®—75 µm, Prolim®—80 µm, BiOSS LIM®—180 µm). StrCSA illustrates differences between the analyzed stents in terms of strut’s geometry even better (Alex®—0.005250 mm2, Prolim®—0.009200 mm2, BiOSS LIM®—0.021600 mm2). Interesting conclusions could also be drawn based on the analysis of the StrCSA calculated for other well-known stents on the market. The following values were obtained: Cypher®—0.019600 mm2, Excel®—0.009520 mm2, Orsiro®—0.003600 mm2, Xience®—0.006561 mm2 and Supralimus®—0.003600 mm2. This calculation definitely shows that the higher the StrCSA is, the bigger LLL value are obtained (Fig. 5).
OCT enabling single stent strut analysis seems to be the best method for tissue response assessment after stent implantation. We found almost complete vessel healing 12 months after the index procedure for all assessed stents, however stent strut coverage was significantly worse for BiOSS LIM® stent compared with others (Alex® 99.2%, Prolim® 98.6%, BiOSS LIM® 95.8%,). It is very likely that the value of BiOSS LIM® strut coverage rate was determined not only by strut thickness itself but by coronary bifurcation as well. It is worth to be stressed that this parameter for Alex® and Prolim® stents was superior to everolimus-eluting stent Xience V® (Abbott Vascular, Santa Clara, CA) and zotarolimus-eluting stent Resolute Integrity® (96.5 and 93.5%, respectively) in the 13-month OCT substudy of the RESOLUTE All Comers trial [22]. On the other hand, OCT substudy of LEADERS trial showed that BP-DES (BioMatrix, strut thickness—112 µm) characterized a more complete stent coverage (99.4%) as compared with DP-DES—Cypher (97.9%) at 9 months follow-up [23]. Really, there is no clear and simple way to assess vessel wall response after stent implantation even with OCT use. There are no doubts that such a healing process is multifactorial.
As mentioned earlier there was no statistically significant difference between Prolim® and Alex® subgroups in terms of mean neointima burden (24.6 ± 8.6 vs. 19.27 ± 8.11%) and neointima volume (28.16 ± 15.10 vs. 24.51 ± 17.64 mm3), while in BiOSS® group these parameters were significantly larger (30.9 ± 6.2% and 44.9 ± 4.9 mm3). This is a strong confirmation that the higher sirolimus concentration and small stent width (and StrCSA in consequence) in Prolim® stent are responsible for that. Values obtained for Prolim® and Alex® subgroups were higher comparing with cobalt-chromium Excel II stent with strut thickness of 88 µm (11.93 ± 6.08 mm3 and 6.77 ± 4.14%) [24]. Interestingly, these values were comparable to those obtained in Cypher stent (26.61 ± 23.06 mm3 and 15 ± 8%) [25]. We believe that higher sirolimus concentration on Cypher stent (1.4 μg/mm2) and the related drug potency explains those differences.
In our study when neointima burden was analyzed the following factors were significant in the univariate analysis: strut width (87.676, 95% CI 30.305–145.048, p = 0.003) and strut thickness (151.185, 95% CI 38.862–263.508, p = 0.009) as well as in the multivariate analysis: strut width (66.406, 95% CI − 1.957 to 134.768, p = 0.04) and postdilatation (− 4.860, 95% CI − 9.982 to 0.263, p = 0.04).
Although it is often thought that OCT is the most accurate technique for analyzing coronary lesions, whilst QCA incurs systematic underestimation [26], in our study we have shown that in all three stents there was a strong correlation between LLL and OCT parameters such as neointima area, neointima volume and neointima burden (Fig. 2). Also, the role of StrCSA were confirmed in OCT imaging. The higher the “product” was, the bigger neointima burden and neointima volume were obtained (Fig. 4; Tables 6, 7).
We strongly believe that the proposed new parameter might be clinically relevant, however needs to be evaluated in a properly designed prospective study.
Ultimately, it is worth mentioning the OCT results which provided the additional insight in the characteristics of neointima formation. In most cases homogenous peristrut or preexisting atheroma was observed in all subgroups (Alex®—87%, Prolim®—83%, BiOSS®—73%). The highest rate of homogenous pattern was observed in the Alex while the lowest in the BiOSS LIM® stent. Probably due to the small number of cases these differences were not statistically significant, however it suggests that StrCSA value stays in relation with neointimal proliferation pattern. In other words the most favorable profile was obtained in case of Alex® stent, a little bit worse in Prolim® stent i.e. in both fulfilling criterion of new generation DES. We found that also for thick strut BiOSS LIM® stent this rate was lower comparing with Cypher stent (65%). This latter stent had a higher sirolimus concentration and considered as toxic mixture of polymers [25]. These abovementioned data are crucial since the homogeneous neointima pattern correlated in earlier reports with a high proportion of connective tissue and smooth muscle cells in histopathology indicating favorable vessel healing, whereas, heterogenous neointima was found to correlate with higher presence of fibrin as compared to homogenous one and was associated with poorer clinical outcomes [27].
Study limitations
This registry has several limitations that should be acknowledged. First, the sample size was relatively small and no sample size calculation was performed. Other limitations of this study are its non-randomized manner and all known drawbacks of registry. Also, the significance of the new parameter ‘strut-cross sectional area’ should be verified in a larger group.
Conclusions
In the QCA and OCT comparative analysis of three DES (Alex®, Prolim® and BiOSS®) we found similar results for the first two, whereas a more pronounced response from the vessel wall was found in the BiOSS® subgroup. Theoretically, the striking lack of differences between Alex® and Prolim® stents might be easily explained by a higher sirolimus concentration and more precise analysis of strut’s geometry represented by the proposed parameter—StrCSA that takes into account not only strut thickness but also its width.
References
Kastrati A, Mehilli J, Dirschinger J, Dotzer F, Schuhlen H, Neumann FJ, Fleckenstein M, Pfafferott C, Seyfarth M, Schomig A (2001) Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation 103(23):2816–2821
Pache J, Kastrati A, Mehilli J, Schuhlen H, Dotzer F, Hausleiter J, Fleckenstein M, Neumann FJ, Sattelberger U, Schmitt C et al (2003) Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. JACC 41(8):1283–1288
Briguori C, Sarais C, Pagnotta P, Liistro F, Montorfano M, Chieffo A, Sgura F, Corvaja N, Albiero R, Stankovic G et al (2002) In-stent restenosis in small coronary arteries: impact of strut thickness. JACC 40(3):403–409
Rittersma SZ, de Winter RJ, Koch KT, Bax M, Schotborgh CE, Mulder KJ, Tijssen JG, Piek JJ (2004) Impact of strut thickness on late luminal loss after coronary artery stent placement. Am J Cardiol 93(4):477–480
Windecker S, Serruys PW, Wandel S, Buszman P, Trznadel S, Linke A, Lenk K, Ischinger T, Klauss V, Eberli F et al (2008) Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet 372(9644):1163–1173
Legutko J, Gil R, Buszman P, Kaluza G, Krol M, Wojdyla R, Pawlowski T, Brzezinski M, Kondys M, Skwarna B et al (2013) OCT evaluation of the time course of vessel healing following implantation of new generation biodegradable polymer-coated and sirolimus-eluting cobalt-chromium coronary stent system (ALEX OCT Study). JACC 62(18/SupplB):170–171
Bil J, Gil RJ, Kern A, Pawlowski T, Seweryniak P, Sliwinski Z (2015) Novel sirolimus-eluting stent Prolim(R) with a biodegradable polymer in the all-comers population: one year clinical results with quantitative coronary angiography and optical coherence tomography analysis. BMC Cardiovasc Disord 15(1):150
Gil RJ, Bil J, Vassiliev D, Inigo Garcia LA (2015) First-in-man study of dedicated bifurcation sirolimus-eluting stent: 12-month results of BiOSS LIM(R) Registry. J Interv Cardiol 28(1):51–60
Milewski K, Gorycki B, Buszman PP, Jelonek M, Beaudry D, Lapointe JM, Guy LG, Abusamra M, Pajak J, Kinasz W et al (2012) Vascular response and mechanical integrity of the new biodegradable polymer coated sirolimus-eluting PROLIM stent implanted in porcine coronary arteries. Kardiol Pol 70(7):703–711
Gil RJ, Bil J, Grundeken MJ, Kern A, Inigo Garcia LA, Vassilev D, Pawlowski T, Formuszewicz R, Dobrzycki S, Wykrzykowska JJ et al (2016) Regular drug-eluting stents versus the dedicated coronary bifurcation sirolimus-eluting BiOSS LIM(R) stent: the randomised, multicentre, open-label, controlled POLBOS II trial. EuroIntervention 12(11):e1404–e1412
Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P et al (2014) 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 35(37):2541–2619
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Jaffe AS, Katus HA, Lindahl B, Morrow DA et al (2012) Third universal definition of myocardial infarction. Circulation 126(16):2020–2035
Vranckx P, Kint PP, Morel MA, Van Es GA, Serruys PW, Cutlip DE (2008) Identifying stent thrombosis, a critical appraisal of the academic research consortium (ARC) consensus definitions: a lighthouse and as a toe in the water. EuroIntervention 4(Suppl C):C39–C44
Bil J, Gil RJ, Vassilev D, Rzezak J, Kulawik T, Pawlowski T (2014) Dedicated bifurcation paclitaxel-eluting stent BiOSS Expert(R) in the treatment of distal left main stem stenosis. J Interv Cardiol 27(3):242–251
Pawlowski T, Prati F, Kulawik T, Ficarra E, Bil J, Gil R (2013) Optical coherence tomography criteria for defining functional severity of intermediate lesions: a comparative study with FFR. Int J Cardiovasc Imaging 29(8):1685–1691
Ali ZA, Roleder T, Narula J, Mohanty BD, Baber U, Kovacic JC, Mintz GS, Otsuka F, Pan S, Virmani R et al (2013) Increased thin-cap neoatheroma and periprocedural myocardial infarction in drug-eluting stent restenosis: multimodality intravascular imaging of drug-eluting and bare-metal stents. Circ Cardiovasc Interv 6(5):507–517
Nagoshi R, Shinke T, Otake H, Shite J, Matsumoto D, Kawamori H, Nakagawa M, Kozuki A, Hariki H, Inoue T et al (2013) Qualitative and quantitative assessment of stent restenosis by optical coherence tomography: comparison between drug-eluting and bare-metal stents. Circ J 77(3):652–660
Mehilli J, Byrne RA, Tiroch K, Pinieck S, Schulz S, Kufner S, Massberg S, Laugwitz KL, Schomig A, Kastrati A et al (2010) Randomized trial of paclitaxel- versus sirolimus-eluting stents for treatment of coronary restenosis in sirolimus-eluting stents: the ISAR-DESIRE 2 (intracoronary stenting and angiographic results: drug eluting stents for in-stent restenosis 2) study. JACC 55(24):2710–2716
Han Y, Jing Q, Chen X, Wang S, Ma Y, Liu H, Luan B, Wang G, Li Y, Wang Z et al (2008) Long-term clinical, angiographic, and intravascular ultrasound outcomes of biodegradable polymer-coated sirolimus-eluting stents. Catheter Cardiovasc Interv 72(2):177–183
Windecker S, Haude M, Neumann FJ, Stangl K, Witzenbichler B, Slagboom T, Sabate M, Goicolea J, Barragan P, Cook S et al (2015) Comparison of a novel biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: results of the randomized BIOFLOW-II trial. Circ Cardiovasc Interv 8(2):e001441
Dani S, Kukreja N, Parikh P, Joshi H, Prajapati J, Jain S, Thanvi S, Shah B, Dutta JP (2008) Biodegradable-polymer-based, sirolimus-eluting Supralimus stent: 6-month angiographic and 30-month clinical follow-up results from the series I prospective study. EuroIntervention 4(1):59–63
Gutierrez-Chico JL, van Geuns RJ, Regar E, van der Giessen WJ, Kelbaek H, Saunamaki K, Escaned J, Gonzalo N, di Mario C, Borgia F et al (2011) Tissue coverage of a hydrophilic polymer-coated zotarolimus-eluting stent vs. a fluoropolymer-coated everolimus-eluting stent at 13-month follow-up: an optical coherence tomography substudy from the RESOLUTE All Comers trial. Eur Heart J 32(19):2454–2463
Barlis P, Regar E, Serruys PW, Dimopoulos K, van der Giessen WJ, van Geuns RJ, Ferrante G, Wandel S, Windecker S, van Es GA et al (2010) An optical coherence tomography study of a biodegradable vs. durable polymer-coated limus-eluting stent: a LEADERS trial sub-study. Eur Heart J 31(2):165–176
Wang G, Sun Z, Jin Q, Xu K, Li Y, Wang X, Ma Y, Liu H, Zhao X, Wang B et al (2015) First-in-man study evaluating the safety and efficacy of a second generation biodegradable polymer sirolimus-eluting stent in the treatment of patients with de novo coronary lesions: clinical, angiographic, and OCT outcomes of CREDIT-1. Catheter Cardiovasc Interv 85(Suppl 1):744–751
Kubo T, Akasaka T, Kozuma K, Kimura K, Kawamura M, Sumiyoshi T, Ino Y, Morino Y, Tanabe K, Kadota K et al (2015) Comparison of neointimal coverage between everolimus-eluting stents and sirolimus-eluting stents: an optical coherence tomography substudy of the RESET (randomized evaluation of sirolimus-eluting versus everolimus-eluting stent trial). EuroIntervention 11(5):564–571
Gutierrez-Chico JL, Serruys PW, Girasis C, Garg S, Onuma Y, Brugaletta S, Garcia-Garcia H, van Es GA, Regar E (2012) Quantitative multi-modality imaging analysis of a fully bioresorbable stent: a head-to-head comparison between QCA, IVUS and OCT. Int J Cardiovasc Imaging 28(3):467–478
Kim JS, Afari ME, Ha J, Tellez A, Milewski K, Conditt G, Cheng Y, Hua Yi G, Kaluza GL, Granada JF (2014) Neointimal patterns obtained by optical coherence tomography correlate with specific histological components and neointimal proliferation in a swine model of restenosis. Eur Heart J Cardiovasc Imaging 15(3):292–298
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RJG was the consultant of Balton company. All the other authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gil, R.J., Bil, J., Legutko, J. et al. Comparative assessment of three drug eluting stents with different platforms but with the same biodegradable polymer and the drug based on quantitative coronary angiography and optical coherence tomography at 12-month follow-up. Int J Cardiovasc Imaging 34, 353–365 (2018). https://doi.org/10.1007/s10554-017-1251-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-017-1251-7