Abstract
Purpose
Colorectal cancer (CRC) risk is associated with modifiable lifestyle factors including smoking, physical inactivity, Western diet, and excess body weight. The impact of lifestyle factors on survival is less known. A cohort study was conducted to investigate the combined effects of a healthy lifestyle and body mass index on prognosis following CRC diagnosis.
Methods
Treatment and follow-up data were collected from the patient files of 1098 participants from the Colorectal cancer low-risk study cohort including stage I-III CRC patients. A healthy lifestyle and BMI (HL) score was computed using self-reported data on smoking status, physical activity, adherence to a Mediterranean diet pattern, and BMI, and divided into four categories ranging from least to most healthy. Survival analyses were performed to assess recurrence-free survival and overall survival across categories of exposure, using the Kaplan–Meier method and Cox proportional hazards models adjusted for age, sex, and educational level.
Results
Among 1098 participants with stage I-III CRC, 233 (21.2%) had an HL score of 0–1 (least healthy), 354 (32.2%) HL score of 2, 357 (32.5%) HL score of 3 and 154 (14.0) HL score 4 (most healthy). Patients with the healthiest lifestyle (HL score 4) compared to the least healthy (HL score 0–1) had an improved recurrence-free survival (HL 4 vs HL 0–1, HRadj 0.51 (95% CI 0.31–0.83) and overall survival (HL 4 vs HL 0–1, HRadj 0.52 (95% CI 0.38–0.70).
Conclusion
Adherence to a healthy lifestyle may increase the recurrence-free and overall survival of patients with stage I–III CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Colorectal cancer (CRC) is the third most common cancer globally, with incidence rates that positively correlate with Human Development Index levels (HDI), a measure of societal development based on the average health, education, and income of a population [1, 2]. Several CRC risk factors are associated with socioeconomic development including smoking, physical inactivity, unhealthy diets, and excess body weight [3]. Incidence rates are declining among older adults in high-income countries due to screening and early removal of precursor lesions but increasing among young adults (age < 50 years) [4]. The reason for this increase is unknown, but lifestyle exposures in childhood and adolescence are considered drivers [5]. Economically transitioning countries are seeing a rapid increase in CRC incidence, albeit from low levels, reflecting a global shift toward more Westernized lifestyles [6].
Less is known about the impact of lifestyle factors on CRC prognosis. Smoking [7, 8], physical inactivity [9, 10], and unhealthy diets [11, 12] have all been associated with increased mortality in CRC patients. The impact of overweight and obesity on CRC survival is, however, highly debated [13]. Some studies have reported improved survival among CRC patients with excess body weight compared to normal weight patients [14], the so-called obesity paradox [15]. Others report worse CRC-specific outcomes in the obese group [16, 17], and yet other studies find no associations between body mass index (BMI) and survival [18]. Reverse causality due to illness-induced weight loss may account for these differences, highlighting the importance of timing when assessing body weight [19].
Few studies have examined the combined effects of lifestyle factors on CRC recurrence and CRC-specific survival [20,21,22,23]. Previous studies have reported conflicting results, which may reflect methodological differences, including the timing of exposure assessment (pre/post-diagnosis), the use of different lifestyle scores, and differences in study populations. This study aimed to investigate the associations of the combined impact of pre-diagnostic modifiable healthy lifestyle factors, including avoidance of smoking, moderate to high levels of physical activity, high adherence to a healthy diet, and BMI within the healthy range, with CRC recurrence and overall survival in a cohort of Swedish CRC stage I-III patients.
Methods
Study design
The Colorectal cancer low-risk study cohort consists of more than 3300 participants diagnosed with all-stage CRC in 14 hospitals in Middle Sweden from 2003 to 2009, as described elsewhere [24]. Participants were consecutively recruited or identified using data provided by Regional Oncologic Centers. The latter received letters of invitation to participate in the study, and those interested were contacted over the telephone for informed consent and inclusion. A subset of participants included in 2004–2006 received a self-administered questionnaire on lifestyle habits (n = 1767), with a response rate of 93% (n = 1639).
We conducted a cohort study including participants from the Colorectal cancer low-risk cohort with stage I–III CRC who had completed the lifestyle questionnaire. A healthy lifestyle was the exposure of interest and recurrence-free survival (RFS) was the primary outcome of this study, using overall survival (OS) as a secondary outcome.
Participants
Participants with a radically resected adenocarcinoma of the colon or rectum that had surgery in 2003–2006 were eligible for inclusion. Stage IV CRC patients were excluded due to dismal prognosis, as were participants with unavailable patient files or missing data on the American Joint Committee of Cancer (AJCC) TNM stage. Patients were staged according to version 5 of the AJCC TNM [25].
Two investigators (S.B and P.R) collected treatment and follow-up data for the participants during the years 2017–2020, including date of surgery, American Society of Anesthesiologists (ASA) classification, oncological treatment (neoadjuvant radiotherapy/adjuvant chemotherapy), time to CRC recurrence, time to last recurrence-free follow-up visit, and time to all-cause death.
Exposure assessment
A semiquantitative questionnaire was used for the collection of information on smoking, physical activity, and anthropometric markers. Participants were asked to report their cigarette smoking status and history including the number of cigarettes per day and duration of smoking for current and ever-smokers. Data on physical activity including leisure time exercise was collected using a validated set of questions with 5 pre-defined duration categories ranging from less than 1 h to more than 5 h/week, a validated method for assessing physical activity [26]. Self-reported weight 5 years before diagnosis was used to calculate BMI by dividing the weight in kilograms by the square of height in meters. Weight 5 years prior to diagnosis was chosen to minimize the risk of reverse causation, as CRC can induce weight loss.
The lifestyle questionnaire included a food frequency section designed to assess a typically Swedish diet. Participants were asked to report serving size and average intake frequency of 96 commonly eaten foods and beverages 5 years before diagnosis. A similar validated questionnaire, where participants report eating habits over the last year, has been used in previous studies [27, 28].
Mediterranean diet score
The Mediterranean diet (MD) is one of the most scientifically evaluated dietary patterns in the field of nutritional epidemiology [29, 30]. Several studies have reported inverse associations between MD adherence and CRC risk and mortality [31,32,33,34,35]. A diet adhering to the MD pattern was considered healthy in this study.
We used the modified Mediterranean diet scale (mMED) defined by Tektonidis et al. and developed further by Larsson et. al to compute a diet variable [36, 37]. This is a modification of the Mediterranean diet scale originally constructed by Trichopoulou, to better suit the intake habits of the Swedish population. The mMED score was created by categorizing the intakes of the following 6 food groups into quintiles: vegetables and fruits, legumes and nuts, whole grains, fish, dairy products, and red and processed meats. Participants received a score from 1 to 5 for being in the first five groups’ lowest to highest quintiles of intake. The score was reversed for the last group, red and processed meats, assigning 5 points to the lowest quintile. The use of olive- or rapeseed oil was assigned 5 points; conversely, 1 point was assigned for non-use. The mMED has previously included intakes of alcoholic beverages. However, the health effects of moderate alcohol consumption are widely debated [38], prompting us to exclude alcohol consumption from the score. The total mMED score thus ranged from 7 (low adherence) to 35 (high adherence).
Healthy lifestyle and BMI score
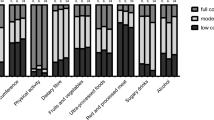
A healthy lifestyle and BMI (HL) score was created by dichotomizing each of the four lifestyle variables into a pre-defined healthy and less healthy/unhealthy alternative [39]. Never smokers and former smokers with > 1 year of cessation time were considered non-smokers, as opposed to current and former smokers with ≤ 1 year of cessation. Current but not former smoking has been associated with poorer CRC-specific survival [7]. According to the WHO recommendations for adults, participants with ≥ 150 min/week of leisure time exercise were considered physically active, versus < 150 min/week [40]. A low-risk diet was defined as an mMED score > the cohort median, versus an mMED score ≤ cohort median. Participants with a BMI of 18.5–24.9 were considered to have healthy body weight, as opposed to those with underweight (BMI < 18.5) and pre-obesity or obesity (BMI ≥ 25.0 m2), according to the WHO classification [41]. One point was allocated for each healthy lifestyle factor, and 0 points for the less healthy or unhealthy alternative. The total score thus ranged from 4 (most adherent to a healthy lifestyle) to 0 (least adherent).
Outcome assessment
CRC recurrence was defined as locoregional recurrence, distant metastasis, or the occurrence of a new colorectal tumor. Observation time started on the date of curative surgery and ended on recurrence or the date of the last follow-up visit to the surgical or oncological clinic. In the OS analysis, participants were observed from the date of curative surgery to the date of all-cause death or the last known date of contact.
Statistical methods
We categorized participants into four groups based on HL points. Those with an HL score of 0 and 1 were combined into one group due to low numbers in the former category (n = 20). Those missing data on smoking (0.8%) were coded as non-smokers. Participants who had left the entire diet section of the FFQ blank were considered non-responders and excluded (0.9%). Median imputation was used to replace missing values for single food groups (6.5%), physical activity (2.8%), and BMI (2.6%).
The distribution of demographic variables across categories of exposure was tested using the chi-square test for categorical and the Kruskal–Wallis test for continuous variables. We used the Kaplan–Meier (K-M) method to assess median RFS and OS in each group and Cox proportional hazards model analysis to estimate univariate and multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for CRC recurrence and all-cause death. The survival analysis was right censored. The least healthy group served as the reference category.
The pre-defined confounders age, sex, and educational level were included in the multivariable model (Figure S1). Tumor stage, oncological treatment, and tumor site were considered potential mediators of the effect of a healthy lifestyle on RFS (Figure S1). Diabetes and cardiovascular disease (CVD) were considered potential mediators of the effect of a healthy lifestyle on OS (Figure S2).
Using the Wald test, we tested for interactions between the HL score and tumor site, oncological treatment, and tumor stage. Since most participants with rectal cancer had received neoadjuvant radiotherapy (RT) before surgery, that is before the start of observation time, we analyzed rectal cancer patients separately using an RT variable as a covariate in the multivariate regression model. A complete cases-only analysis was conducted excluding those missing data on any of the HL score variables.
All analyses were done using SPSS version 28.
Results
Participants missing an exact date of recurrence (n = 29), with recurrences occurring ≤ 6 months of diagnosis (n = 18) or follow-up time ≤ 6 months (n = 11) were excluded from the RFS analysis (n =58 ). A total of 1040 participants were included in the RFS analysis and all 1098 participants were included in the OS analysis (Fig. 1).
Flow diagram of participants illustrating the study design according to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations. N number, CRC colorectal cancer, tumor stage = stage according to AJCC TNM 5th edition, R1 microscopically positive resection margins, RFS recurrence-free survival, OS overall survival, m months
Demographic characteristics of participants are shown for the total population and by HL score category in Table 1.
The group with the healthiest lifestyle (HL 4) consisted of 157 participants (14%). These were more likely to be women, of higher age, with a higher educational level, and less often diabetics, as compared to the 233 participants (21%) in the least healthy group (HL 0–1) who were predominantly male and tended to be younger at CRC onset. There were no differences in cancer stage, tumor site, oncological treatment, or other clinical factors. The composition of the HL score is further outlined in Table 2.
We observed 221 events of cancer recurrence among 1040 participants during a median follow-up time of 4.3 years (Fig. 2).
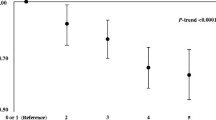
A healthy lifestyle was associated with improved RFS. The crude and adjusted HRs of recurrence and death were significantly lower than the reference for all score categories above the reference. Compared to participants with an HL 0–1 (least healthy), the HL 4 (most healthy) category had a crude HR for recurrence of 0.51 (95% CI 0.32–0.81) and an adjusted HR for recurrence of 0.51 (95% CI 0.31–0.83), with sex, age, and educational level included in the multivariate model. The adjusted HRs for recurrence of participants with an HL 2 and HL 3 were 0.57 (95% CI 0.40–0.81) and 0.66 (0.47–0.92), respectively (Table 3). There were 542 deaths among 1098 participants during a median follow-up time of 6.3 years (Fig. 2). The crude HR for all-cause death for participants with HL 4 vs HL 1 was 0.65 (95% CI 0.48–0.87) and the adjusted HR for all-cause death was 0.52 (95% CI 0.38–0.70) (Table 3). The adjusted HRs for death of HL 2 and 3 vs HL 1 were 0.66 (0.50–0.79) and 0.72 (0.57–0.90). We found no significant interactions between the covariates included in the multivariate model when using the Wald test.
In the K-M curves for recurrence (Fig. 2), the curve of the least healthy group was found to have a disproportional course in relation to the others with events occurring sooner in this group, indicating changes in HR over time. The proportional hazards assumption was not valid (Log-Rank test p-value = 0.001). The HRs for recurrence are thus to be interpreted as average estimates for the whole time of observation.
Sensitivity analysis
The violation of the proportional hazards assumption prompted us to conduct a Cox proportional hazards analysis with time-dependent covariates, in order to estimate the HRs for time periods < 24 months, ≥ 24 months – < 36 months, ≥ 36 months – < 48 months, and ≥ 48 months – < 60 months. The strongest effect of the HL score on RFS was seen in the interval of ≥ 24 months – < 36 months (Table S1).
We found no significant interaction effects between the HL score and cancer stage (p-value: 0.39), tumor location (p-value 0.68), and oncological treatment (p-value:0.66) when using the Wald test in the RFS analysis, A complete cases-only analysis was performed, excluding all cases with missing values in score components. This only slightly affected estimated HRs and 95% CIs (Table S2).
We analyzed the rectal cancer group separately, including a covariate for radiotherapy in the Cox model. Participants had received a total dose of either 25 Gy (80% of those treated with neoadjuvant RT) or 50,4 Gy. We coded a categorical variable with three levels (0 = no RT, 1 = 25 Gy, 2 = 50.4 Gy), and tested it in a Cox model for rectal cases only. The p-value of the RT-covariate was non-significant.
When including the individual score components as covariates in a multivariate Cox regression analysis, only smoking, and exercise were significantly associated with a reduced HR of CRC recurrence and death (Table S3). Sex, age, and level of education were all significantly associated with OS, but not RFS.
Discussion
In this cohort study, patients with CRC stage I-III and a healthy lifestyle (HL 4) had a 49% lower HR of cancer recurrence and a 48% lower HR of all-cause death compared with the least healthy (HL 1). As we found the proportional hazards assumption to be violated in our survival analyses, indicating changes in hazard rate over time, we investigated the time-varying effects of the score on RFS. The results indicate a stronger effect in the interval of 24–36 months. However, only a small number of recurrences occurred after this period and the results for survival > 36 months should thus be interpreted with caution.
This is one of the first studies to report a statistically significant decrease in the risk of CRC recurrence in patients adhering to a healthy lifestyle pre-diagnosis. A small number of previous studies have reported inverse associations between a healthy lifestyle and overall mortality, but results for RFS or CRC-specific mortality have often been weaker.
Among 5727 all-stage CRC cases, Pelser et al. reported a statistically significant reduction in the risk of all-cause death for those with a pre-diagnostic healthy lifestyle (including a BMI within the normal range, smoking avoidance, physical activity, a healthy diet, and a low intake of alcohol) [20]. Reduced risk of CRC-specific death was seen only among rectal cancer cases, whereas our results indicate a protective effect of a healthy lifestyle irrespective of the anatomical subsite.
In a cohort of CRC patients from the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS), pre-and post-diagnostic adherence to the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) score was significantly associated with a lower risk of all-cause, but not CRC-specific, death [21]. This score includes physical activity, diet, and body weight, but not smoking. Non-smoking was our study’s strongest individual risk-reducing factor, which may account for the differences. Among 3292 cases of all-stage CRC within the European Prospective Investigation into Cancer and Nutrition (EPIC) study, pre-diagnostic concordance with the WCRF/AICR recommendations was however associated with reduced CRC-related and overall mortality [22].
Among 992 colon cancer stage III cases, finally, a healthy lifestyle post-diagnosis (including normal body weight maintenance, physical activity, and a healthy diet) according to the guidelines issued by the American Cancer Society (ACS) was associated with a significant improvement in OS and a significant trend toward improved RFS over a 7-year median follow-up time [23]. Stratifying for tumor stage did not indicate stage-specific associations between lifestyle and survival in our study.
A recently published large study on the associations between healthy lifestyles and cancer morbidity and mortality in diabetics, including 1904 participants with CRC, found a 45% lower risk of cancer mortality among those with the healthiest lifestyle, compared to the least healthy [42]. Lifestyle and dietary factors have also been included in recurrence and survival prediction models for colon cancer stage III, resulting in significantly improved predictions [43].
The suggested biological mechanisms conferring a protective effect of a healthy lifestyle on CRC risk include decreases in inflammation and oxidative stress, modulation of gut microbiota, decreased bowel transit time, and increases in insulin sensitivity [44,45,46]. The same mechanisms may be involved in reducing the risk of recurrence. Traditional models of tumorigenesis have considered systemic tumor spread to be a late event in the process of primary tumor progression. This is being challenged by studies showing that dissemination can occur also in the early stages of this process [47, 48] even in preneoplastic lesions [49]. Environmental exposures during the process of tumorigenesis could thus influence the risk of dissemination.
We’ve used a diet score modified to suit the intakes of a Swedish population, which may impair the generalizability of our results. However, the other lifestyle variables were assessed using internationally established criteria and the results may thus apply to other high-income or even transitioning populations. Our results indicate that pre-diagnosis lifestyle has an impact not only on CRC risk but also on disease-specific survival, which underlines the importance of primary preventive measures. Further studies are warranted to confirm our results.
Strengths and limitations
Our study has several strengths including a long follow-up time with many observed events, detailed clinical data, and a design that may have reduced the risk of reverse causation in lifestyle assessment. The study is based on high-quality questionnaires, and the method has been evaluated previously. Further, the proportion of questionnaire responders was high (93%) decreasing the risk of selection bias and missing data was scarce, increasing the internal validity. There are also weaknesses to consider, including the risk of misclassification bias in self-reported data. Unmeasured lifestyle changes post-diagnosis may be reflected in our results. Studies on lifestyle changes in CRC survivors report conflicting results, with some finding shifts towards more healthy dietary habits [50], and smoking cessation [51], while others report little or no change in lifestyle [52].
Our exposure variable, the HL score, is based on four dichotomized lifestyle factors, each given equal weight within the score. Our results however indicate that non-smoking and physical activity have a stronger association with an improved recurrence-free and overall survival than a healthy diet and BMI within the normal range. Future studies could thus consider using a weighted score. We chose to dichotomize the BMI variable, placing the underweight participants in the same “unhealthy” category as the overweight and obese. The impact of underweight, overweight, and obesity on CRC recurrence and survival may however differ, which should be considered in future studies. Using additional anthropometric markers may further improve body weight assessment [6]. Confounding due to additional unmeasured factors cannot be ruled out.
Conclusions
Our study indicates that adherence to a healthy lifestyle may increase the RFS and OS of patients with stage I-III CRC. Avoidance of smoking and being physically active were independent risk-reducing factors for these outcomes.
Data availability
The datasets analysed during the current study include sensitive and detailed data on the health and habits of study participants. They are not publicly available due to the risk of compromising their anonymity, but available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Klugman J, Rodriguez F, Choi H-J (2011) The HDI 2010: new controversies, old critiques UNDP (United Nations Development Programme). J Econ Inequal 9:249–288
WCRF/AICR (2018) Diet, nutrition, physical activity and cancer: a global perspective. Continuous Update Project Expert Report
Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M et al (2020) Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. https://doi.org/10.1136/gutjnl-2022-327736
Dharwadkar P, Zaki TA, Murphy CC (2022) Colorectal cancer in younger adults. Hematol Oncol Clin North Am 36(3):449–470. https://doi.org/10.1016/j.hoc.2022.02.005
Keum N, Giovannucci E (2019) Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol 16(12):713–732. https://doi.org/10.1038/s41575-019-0189-8
Alwers E, Carr PR, Banbury B, Walter V, Chang-Claude J, Jansen L et al (2021) Smoking behavior and prognosis after colorectal cancer diagnosis: a pooled analysis of 11 studies. JNCI Cancer Spectr. https://doi.org/10.1093/jncics/pkab077
Ordonez-Mena JM, Walter V, Schottker B, Jenab M, O’Doherty MG, Kee F et al (2018) Impact of prediagnostic smoking and smoking cessation on colorectal cancer prognosis: a meta-analysis of individual patient data from cohorts within the CHANCES consortium. Ann Oncol 29(2):472–483. https://doi.org/10.1093/annonc/mdx761
Schmid D, Leitzmann MF (2014) Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol 25(7):1293–1311. https://doi.org/10.1093/annonc/mdu012
Van Blarigan EL, Meyerhardt JA (2015) Role of physical activity and diet after colorectal cancer diagnosis. J Clin Oncol 33(16):1825–1834. https://doi.org/10.1200/JCO.2014.59.7799
Zhu Y, Wu H, Wang PP, Savas S, Woodrow J, Wish T et al (2013) Dietary patterns and colorectal cancer recurrence and survival: a cohort study. BMJ Open. https://doi.org/10.1136/bmjopen-2012-002270
Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Hu FB, Mayer RJ et al (2007) Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA 298(7):754–764. https://doi.org/10.1001/jama.298.7.754
van Zutphen M, Kampman E, Giovannucci EL, van Duijnhoven FJB (2017) Lifestyle after colorectal cancer diagnosis in relation to survival and recurrence: a review of the literature. Curr Colorectal Cancer Rep 13(5):370–401. https://doi.org/10.1007/s11888-017-0386-1
Walter V, Jansen L, Hoffmeister M, Ulrich A, Roth W, Blaker H et al (2016) Prognostic relevance of prediagnostic weight loss and overweight at diagnosis in patients with colorectal cancer. Am J Clin Nutr 104(4):1110–1120. https://doi.org/10.3945/ajcn.116.136531
Banack HR, Stokes A (2017) The ‘obesity paradox’ may not be a paradox at all. Int J Obes (Lond) 41(8):1162–1163. https://doi.org/10.1038/ijo.2017.99
Jaspan V, Lin K, Popov V (2021) The impact of anthropometric parameters on colorectal cancer prognosis: a systematic review and meta-analysis. Crit Rev Oncol Hematol 159:103232. https://doi.org/10.1016/j.critrevonc.2021.103232
Kohls M, Freisling H, Charvat H, Soerjomataram I, Viallon V, Davila-Batista V et al (2022) Impact of cumulative body mass index and cardiometabolic diseases on survival among patients with colorectal and breast cancer: a multi-centre cohort study. BMC Cancer 22(1):546. https://doi.org/10.1186/s12885-022-09589-y
Boyle T, Fritschi L, Platell C, Heyworth J (2013) Lifestyle factors associated with survival after colorectal cancer diagnosis. Br J Cancer 109(3):814–822. https://doi.org/10.1038/bjc.2013.310
Silva A, Faria G, Araujo A, Monteiro MP (2020) Impact of adiposity on staging and prognosis of colorectal cancer. Crit Rev Oncol Hematol 145:102857. https://doi.org/10.1016/j.critrevonc.2019.102857
Pelser C, Arem H, Pfeiffer RM, Elena JW, Alfano CM, Hollenbeck AR et al (2014) Prediagnostic lifestyle factors and survival after colon and rectal cancer diagnosis in the National Institutes of Health (NIH)-AARP diet and health Study. Cancer 120(10):1540–1547. https://doi.org/10.1002/cncr.28573
Song R, Petimar J, Wang M, Tabung FK, Song M, Liu L et al (2021) Adherence to the World cancer research Fund/American Institute for cancer research cancer prevention recommendations and colorectal cancer survival. Cancer Epidemiol Biomarkers Prev 30(10):1816–1825. https://doi.org/10.1158/1055-9965.Epi-21-0120
Romaguera D, Ward H, Wark PA, Vergnaud AC, Peeters PH, van Gils CH et al (2015) Pre-diagnostic concordance with the WCRF/AICR guidelines and survival in European colorectal cancer patients: a cohort study. BMC Med 13:107. https://doi.org/10.1186/s12916-015-0332-5
Van Blarigan EL, Fuchs CS, Niedzwiecki D, Zhang S, Saltz LB, Mayer RJ et al (2018) Association of survival with adherence to the American Cancer Society Nutrition and physical activity guidelines for cancer survivors after colon cancer diagnosis: The CALGB 89803/Alliance trial. JAMA Oncol 4(6):783–790. https://doi.org/10.1001/jamaoncol.2018.0126
Forsberg A, Keranen A, Vonh S, Picelli S, Papadogiannakis N, Ghazi S, et al (2017) Defining new colorectal cancer syndromes in a population-based cohort of the disease. Anticancer Res 37(4):1831–1835. https://doi.org/10.21873/anticanres.11518
AJCC (1997) AJCC Cancer Staging Manual - Fifth Edition Philadelphia: Lippincott - Raven Publishers
Orsini N, Bellocco R, Bottai M, Hagstromer M, Sjostrom M, Pagano M et al (2008) Validity of self-reported total physical activity questionnaire among older women. Eur J Epidemiol 23(10):661–667. https://doi.org/10.1007/s10654-008-9273-z
Messerer M, Johansson SE, Wolk A (2004) The validity of questionnaire-based micronutrient intake estimates is increased by including dietary supplement use in Swedish men. J Nutr 134(7):1800–1805. https://doi.org/10.1093/jn/134.7.1800
Rautiainen S, Serafini M, Morgenstern R, Prior RL, Wolk A (2008) The validity and reproducibility of food-frequency questionnaire-based total antioxidant capacity estimates in Swedish women. Am J Clin Nutr 87(5):1247–1253. https://doi.org/10.1093/ajcn/87.5.1247
Martinez-Gonzalez MA, Trichopoulou A (2020) Observational epidemiology, lifestyle, and health: The Paradigm of the Mediterranean diet. Am J Health Promot 34(8):948–950. https://doi.org/10.1177/0890117120960580c
Trichopoulou A, Costacou T, Bamia C, Trichopoulos D (2003) Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 348(26):2599–2608. https://doi.org/10.1056/NEJMoa025039
Schulpen M, van den Brandt PA (2020) Mediterranean diet adherence and risk of colorectal cancer: the prospective Netherlands Cohort Study. Eur J Epidemiol 35(1):25–35. https://doi.org/10.1007/s10654-019-00549-8
Rosato V, Guercio V, Bosetti C, Negri E, Serraino D, Giacosa A et al (2016) Mediterranean diet and colorectal cancer risk: a pooled analysis of three Italian case-control studies. Br J Cancer 115(7):862–865. https://doi.org/10.1038/bjc.2016.245
Schwingshackl L, Schwedhelm C, Galbete C, Hoffmann G (2017) Adherence to mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients. https://doi.org/10.3390/nu9101063
Castello A, Rodriguez-Barranco M, Fernandez de Larrea N, Jakszyn P, Dorronsoro A, Amiano P et al (2022) Adherence to the Western, prudent and Mediterranean dietary patterns and colorectal cancer risk: findings from the Spanish Cohort of the European prospective investigation into Cancer and nutrition (EPIC-Spain). Nutrients. https://doi.org/10.3390/nu14153085
Veettil SK, Wong TY, Loo YS, Playdon MC, Lai NM, Giovannucci EL et al (2021) Role of diet in colorectal cancer incidence: umbrella review of meta-analyses of prospective observational studies. JAMA Netw Open 4(2):e2037341. https://doi.org/10.1001/jamanetworkopen.2020.37341
Tektonidis TG, Akesson A, Gigante B, Wolk A, Larsson SC (2015) A Mediterranean diet and risk of myocardial infarction, heart failure and stroke: a population-based cohort study. Atherosclerosis 243(1):93–98. https://doi.org/10.1016/j.atherosclerosis.2015.08.039
Larsson SC, Hakansson N, Wolk A (2017) Healthy dietary patterns and incidence of biliary tract and gallbladder cancer in a prospective study of women and men. Eur J Cancer 70:42–47. https://doi.org/10.1016/j.ejca.2016.10.012
Collaborators GBDA (2022) Population-level risks of alcohol consumption by amount, geography, age, sex, and year: a systematic analysis for the Global Burden of Disease Study 2020. Lancet 400(10347):185–235. https://doi.org/10.1016/S0140-6736(22)00847-9
Larsson SC, Akesson A, Wolk A (2015) Primary prevention of stroke by a healthy lifestyle in a high-risk group. Neurology 84(22):2224–2228. https://doi.org/10.1212/WNL.0000000000001637
Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G et al (2020) World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 54(24):1451–1462. https://doi.org/10.1136/bjsports-2020-102955
WHO (1998) Obesity: preventing and managing the global epidemic. Report of a WHO consultation on obesity. Geneva, June 3–5, 1997. Geneva: WHO
Zhang YB, Pan XF, Lu Q, Wang YX, Geng TT, Zhou YF et al (2022) Associations of combined healthy lifestyles with cancer morbidity and mortality among individuals with diabetes: results from five cohort studies in the USA, the UK and China. Diabetologia. https://doi.org/10.1007/s00125-022-05754-x
Cheng E, Ou FS, Ma C, Spiegelman D, Zhang S, Zhou X et al (2022) Diet- and lifestyle-based prediction models to estimate cancer recurrence and death in patients with stage III colon cancer (CALGB 89803/Alliance). J Clin Oncol 40(7):740–751. https://doi.org/10.1200/JCO.21.01784
Wolpin BM, Meyerhardt JA, Chan AT, Ng K, Chan JA, Wu K et al (2009) Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol 27(2):176–185. https://doi.org/10.1200/JCO.2008.17.9945
Mena MP, Sacanella E, Vazquez-Agell M, Morales M, Fito M, Escoda R et al (2009) Inhibition of circulating immune cell activation: a molecular antiinflammatory effect of the Mediterranean diet. Am J Clin Nutr 89(1):248–256. https://doi.org/10.3945/ajcn.2008.26094
Gill CI, Boyd A, McDermott E, McCann M, Servili M, Selvaggini R et al (2005) Potential anti-cancer effects of virgin olive oil phenols on colorectal carcinogenesis models in vitro. Int J Cancer 117(1):1–7. https://doi.org/10.1002/ijc.21083
Welch DR, Hurst DR (2019) Defining the hallmarks of metastasis. Cancer Res 79(12):3011–3027. https://doi.org/10.1158/0008-5472.CAN-19-0458
Turajlic S, Swanton C (2016) Metastasis as an evolutionary process. Science 352(6282):169–175. https://doi.org/10.1126/science.aaf2784
Lambert AW, Pattabiraman DR, Weinberg RA (2017) Emerging biological principles of metastasis. Cell 168(4):670–691. https://doi.org/10.1016/j.cell.2016.11.037
Van Loon K, Wigler D, Niedzwiecki D, Venook AP, Fuchs C, Blanke C et al (2013) Comparison of dietary and lifestyle habits among stage III and metastatic colorectal cancer patients: findings from CALGB 89803 and CALGB 80405. Clin Colorectal Cancer 12(2):95–102. https://doi.org/10.1016/j.clcc.2012.11.002
Skeie G, Hjartaker A, Braaten T, Lund E (2009) Dietary change among breast and colorectal cancer survivors and cancer-free women in the Norwegian Women and Cancer cohort study. Cancer Causes Control 20(10):1955–1966. https://doi.org/10.1007/s10552-009-9390-3
van Zutphen M, Boshuizen HC, Kok DE, van Baar H, Geijsen A, Wesselink E et al (2019) Colorectal cancer survivors only marginally change their overall lifestyle in the first 2 years following diagnosis. J Cancer Surviv 13(6):956–967. https://doi.org/10.1007/s11764-019-00812-7
Acknowledgments
The authors would like to thank all study participants, clinicians, and staff of the Swedish low-risk colorectal cancer study group. The late Berith Wejderot stands out for her diligent work with study inclusion and the handling of questionnaires. Professor Alicja Wolk at the Institute of Environmental Medicine KI contributed to the questionnaire design and Jan-Erik Frödin, MD and Ph.D. at the Department of Onclogy-Pathology KI contributed to the study design and helped plan the collection of data. We would also like to express our gratitude to biostatistician Mikael Andersson Franko at the Department of Clinical Science and Education at Karolinska Institutet for overseeing the data analysis and making valuable contributions to our discussions on the interpretation and generalizability of our results.
Funding
Open access funding provided by Karolinska Institute. This study was supported by Swedish Research Council, grant number 2019-01441, the Stockholm county council ALF project, grant number RS2020-0731, and the Swedish Cancer Society 211443Pj02H.
Author information
Authors and Affiliations
Contributions
SB, PR, UL, SL, AL, and AL contributed to the study design and planning. Data collection was performed by SB and PR. Analyses were performed by SB. The manuscript was drafted by SB and PR and all authors reviewed and commented on previous versions of the manuscript. The final manuscript has been read and approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to disclose.
Ethics approval
This study was approved by the Regional ethical review board in Stockholm (Dnr 02-439, 04-4377, 2009/2155-32, 2013/928-32, 2014/1326-32, 2017/57-31/4).
Consent to participate
Informed content was obtained from all study participants upon inclusion.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barot, S., Rantanen, P., Nordenvall, C. et al. Combined associations of a healthy lifestyle and body mass index with colorectal cancer recurrence and survival: a cohort study. Cancer Causes Control 35, 367–376 (2024). https://doi.org/10.1007/s10552-023-01802-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-023-01802-y