Abstract
Purpose
Patients’ chronic disease burden can influence the likelihood that providers will recommend cancer screening and that patients will participate in it. Using data from the STOP CRC pragmatic study, we examined associations between chronic disease burden and colorectal cancer screening recommendation and use.
Methods
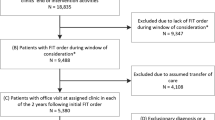
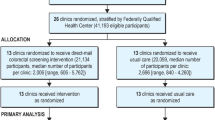
Participating STOP CRC clinics (n = 26) received either usual care or training to implement a mailed fecal immunochemical test (FIT) outreach program. Selected clinic patients (n = 60,187 patients) were aged 50–74 and overdue for colorectal cancer screening. We used logistic regression to examine the associations between FIT recommendations and completion and patients’ chronic disease burden, calculated using the Charlson Comorbidity Index and the Chronic Illness and Disability Payment System.
Results
For each index, FIT recommendation odds were 8–9% higher among individuals with minimal chronic disease burden and 13–23% lower among individuals with high chronic disease burden (inverted U-shaped association). Among adults who were ordered a FIT, FIT completion odds were 20% lower for individuals with any, versus no, chronic condition and diminished with increasing disease burden (inverse linear association).
Conclusions
Analysis showed an inverted U-shaped association between patients’ chronic disease burden and providers’ recommendation of a FIT and an inverse linear association between patients’ chronic disease burden and FIT completion. ClinicalTrials.gov registration: NCT01742065


Similar content being viewed by others
References
American Cancer Society (2019) Cancer Statistics Center.
Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, Stewart ET, Waye JD (2012) Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 366:687–696
Centers for Disease Control and Prevention (2016) Behavioral Risk Factor Surveillance System Survey Data. Atlanta, Georgia Department of Health and Human Services, Centers for Disease Control and Prevention.
American Association for Retired Persons Chronic Conditions Among Older Americans; . In: Anderson G, ed. Analysis of Medical Expenditure Panel Survey data from 2005
Zhao G, Ford ES, Ahluwalia IB, Li C, Mokdad AH (2009) Prevalence and trends of receipt of cancer screenings among US women with diagnosed diabetes. J Gen Intern Med 24:270–275
Heflin MT, Pollak KI, Kuchibhatla MN, Branch LG, Oddone EZ (2006) The impact of health status on physicians’ intentions to offer cancer screening to older women. J Gerontol 61:844–850
Fontana SA, Baumann LC, Helberg C, Love RR (1997) The delivery of preventive services in primary care practices according to chronic disease status. Am J Public Health 87:1,190–1,196
Fox J, Zikmund-Fisher BJ, Gross CP (2012) Older patient experiences in the mammography decision-making process. Arch Intern Med. https://doi.org/10.1001/archinternmed.2011.601
Feuer EJ, Lee M, Mariotto AB, Cronin KA, Scoppa S, Penson DF, Hachey M, Cynkin L, Carter GA, Campbell D, Percy-Laurry A, Zou Z, Schrag D, Hankey BF (2012) The Cancer Survival Query System: making survival estimates from the Surveillance, Epidemiology, and End Results program more timely and relevant for recently diagnosed patients. Cancer 118:5,652–5,662
Guiriguet C, Pera G, Castells A, Toran P, Grau J, Rivero I, Buron A, Macia F, Vela-Vallespin C, Vilarrubi-Estrella M, Marzo-Castillejo M (2017) Impact of comorbid conditions on participation in an organised colorectal cancer screening programme: a cross-sectional study. BMC Cancer 17:524
Diaz A, Kang J, Moore SP, Baade P, Langbecker D, Condon JR, Valery PC (2017) Association between comorbidity and participation in breast and cervical cancer screening: A systematic review and meta-analysis. Cancer Epidemiol 47:7–19
Kronick R, Gilmer T, Dreyfus T, Lee L (2000) Improving health-based payment for Medicaid beneficiaries: CDPS. Health Care Financ Rev 21:29–64
Oemrawsingh A, Swami N, Valderas JM, Hazelzet JA, Pusic AL, Gliklich RE, Bergmark RW (2020) Patient-Reported Morbidity Instruments: A Systematic Review. Value Health 23:791–811. https://doi.org/10.1016/j.jval.2020.02.006 (Epub May 27)
HRSA (2016) 2016 National Health Center Data.
Coronado GD, Petrik AF, Vollmer WM, Taplin SH, Keast EM, Fields S, Green BB (2018) Effectiveness of a Mailed Colorectal Cancer Screening Outreach Program in Community Health Clinics: The STOP CRC Cluster Randomized Clinical Trial. JAMA Intern Med 178:1,174–1,181
Coronado GD, Vollmer WM, Petrik A, Taplin SH, Burdick TE, Meenan RT, Green BB (2014) Strategies and Opportunities to STOP Colon Cancer in Priority Populations: Design of a cluster-randomized pragmatic trial. Contemp Clin Trials 38:344–349
Petrik AF, Green BB, Vollmer WM, Le T, Bachman B, Keast E, Rivelli J, Coronado GD (2016) The validation of electronic health records in accurately identifying patients eligible for colorectal cancer screening in safety net clinics. Fam Pract. https://doi.org/10.1093/fampra/cmw065
Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA (2005) Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43:1,130–1,139
Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613–619
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Haas CB, Phipps AI, Hajat A, Chubak J, Wernli KJ (2019) Time to fecal immunochemical test completion for colorectal cancer screening. Am J Manag Care 25:174–180
Klabunde CN, Zheng Y, Quinn VP, Beaber EF, Rutter CM, Halm EA, Chubak J, Doubeni CA, Haas JS, Kamineni A, Schapira MM, Vacek PM, Garcia MP, Corley DA, Consortium P (2016) Influence of Age and Comorbidity on Colorectal Cancer Screening in the Elderly. Am J Prev Med 51:e67-75
Gross CP, Fried TR, Tinetti ME, Ross JS, Genao I, Hossain S, Wolf E, Lewis CL (2015) Decision-making and cancer screening: a qualitative study of older adults with multiple chronic conditions. J Geriatr Oncol 6:93–100
Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D, Brawley OW, Wender RC (2018) Cancer screening in the United States, 2018: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 68:297–316. https://doi.org/10.3322/caac.21446 (Epub 2018 May 30)
Cho H, Klabunde CN, Yabroff KR, Wang Z, Meekins A, Lansdorp-Vogelaar I, Mariotto AB (2013) Comorbidity-adjusted life expectancy: a new tool to inform recommendations for optimal screening strategies. Ann Intern Med 159:667–676
Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Levin TR, Lieberman D, Robertson DJ (2017) Colorectal Cancer Screening: Recommendations for Physicians and Patients From the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology 153:307–323
Braithwaite D, Walter LC, Izano M, Kerlikowske K (2016) Benefits and Harms of Screening Mammography by Comorbidity and Age: A Qualitative Synthesis of Observational Studies and Decision Analyses. J Gen Intern Med 31:561–572
van Hees F, Saini SD, Lansdorp-Vogelaar I, Vijan S, Meester RG, de Koning HJ, Zauber AG, van Ballegooijen M (2015) Personalizing colonoscopy screening for elderly individuals based on screening history, cancer risk, and comorbidity status could increase cost effectiveness. Gastroenterology 149:1,425–1,437
Haggstrom DA, Klabunde CN, Smith JL, Yuan G (2013) Variation in primary care physicians’ colorectal cancer screening recommendations by patient age and comorbidity. J Gen Intern Med 28:18–24
Liu BY, O’Malley J, Mori M, Fagnan LJ, Lieberman D, Morris CD, Buckley DI, Heintzman JD, Carney PA (2014) The association of type and number of chronic diseases with breast, cervical, and colorectal cancer screening. J Am Board Fam Med 27:669–681
O’Neill TJ, Nguemo JD, Tynan AM, Burchell AN (1999) Antoniou T (2017) Risk of Colorectal Cancer and Associated Mortality in HIV: A Systematic Review and Meta-Analysis. J Acquir Immune Defic Syndr 75:439–447
Mani D, Aboulafia DM (2013) Screening guidelines for non-AIDS defining cancers in HIV-infected individuals. Curr Opin Oncol 25:518–525
Mannheimer S, Friedland G, Matts J, Child C, Chesney M (2002) The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis 34:1,115–1,121
Cobigo V, Ouellette-Kuntz H, Balogh R, Leung F, Lin E, Lunsky Y (2013) Are cervical and breast cancer screening programmes equitable? The case of women with intellectual and developmental disabilities. J Intellect Disabil Res 57:478–488
Guilcher SJ, Lofters A, Glazier RH, Jaglal SB, Voth J, Bayoumi AM (2014) Level of disability, multi-morbidity and breast cancer screening: does severity matter? Prev Med 67:193–198
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute, Award Number UH3CA188640. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The study sponsor had no role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Funding
Research reported in this publication was supported by the National Cancer Institute, Award Number UH3CA188640.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Coronado served as a co-investigator on a study funded by Epigenomics and as a principal investigator on a study funded by Quidel Corporation. Dr. Coronado also served as a scientific advisor for Exact Sciences and Guardant Health. The studies had no influence on the design, conduct, or reporting of the present study. All other authors declare that they have no conflict of interest.
Research involving Human Participants and/or Animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (The Institutional Review Board of Kaiser Permanente Northwest; ClinicalTrials.gov registration: NCT01742065) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The Institutional Review Board of Kaiser Permanente Northwest approved all study activities, and participating clinics ceded human subjects review authority to this IRB.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Coronado, G.D., Nielson, C.M., Keast, E.M. et al. The influence of multi-morbidities on colorectal cancer screening recommendations and completion. Cancer Causes Control 32, 555–565 (2021). https://doi.org/10.1007/s10552-021-01408-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-021-01408-2