Abstract
Purpose
Patients with chemotherapy-induced ovarian function failure (CIOFF) may experience ovarian function recovery (OFR). Earlier, we showed that OFR during treatment with anastrozole impacted the prognosis of hormone receptor-positive (HR+) breast cancer (BC) patients with CIOFF. Here, we present the long-term follow-up results.
Methods
Postmenopausal women with HR+ BC who were 45–57 years of age and received chemotherapy were identified from the phase 3 DATA study (NCT00301457) on the extended use of anastrozole. Eligible patients were categorised into two groups: patients with CIOFF and definitely postmenopausal patients. Patients with CIOFF were monitored for OFR. Disease-free survival (DFS), distant recurrence-free survival (DRFS), and overall survival (OS) were compared between patients with OFR and patients without OFR using multivariable Cox regression analyses, including OFR as a time-dependent covariate. BC-specific mortality (BCSM) was compared between groups using the Fine and Gray method.
Results
This study included 656 patients: 395 patients with CIOFF and 261 definitely postmenopausal patients. OFR occurred in 39 (12%) of 329 patients with CIOFF who were monitored for OFR. The median follow-up time was 13.3 years. Patients with OFR experienced a deterioration in DFS (hazard ratio (HR) = 1.54; 95% confidence interval (CI) 0.85–2.81), DRFS (HR = 1.51; 95% CI 0.73–3.11), OS (HR = 1.64; 95% CI 0.75–3.55), and BCSM (subdistribution HR = 1.98; 95% CI 0.84–4.63) when compared with patients without OFR.
Conclusion
In patients with CIOFF, OFR during treatment with anastrozole was associated with a deterioration in BC outcomes. These findings underscore the importance of adequate ovarian function suppression in this subgroup of patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endocrine therapy is an important aspect of treatment of patients diagnosed with early-stage hormone receptor-positive (HR+) breast cancer (BC). The type of endocrine therapy is primarily based on menopausal status. In premenopausal women, endocrine therapy generally consists of a combination of ovarian function suppression (OFS) and either tamoxifen or an aromatase inhibitor for at least 5 years [1]. In postmenopausal women, aromatase inhibitors, either upfront or sequentially after 2–3 years of tamoxifen, are considered superior to tamoxifen during the first 5 years of treatment [2].
Patients who are premenopausal at diagnosis of BC may become postmenopausal as a result of chemotherapy-induced ovarian function failure (CIOFF) [3,4,5]. In a previous exploratory analysis of the DATA study, a randomised controlled trial evaluating the extended use of anastrozole, we showed that disease outcomes of patients with CIOFF do not differ from those of definitely postmenopausal patients of the same age [6]. Prior studies have however shown that patients with CIOFF may experience ovarian function recovery (OFR) during treatment with an aromatase inhibitor [7,8,9,10,11]. In the previous analysis of the DATA study, we revealed that 12% of patients with CIOFF developed OFR during treatment with anastrozole, even though chemotherapy was given two to three years earlier and patients had a median age of 48 years at randomisation [6, 11]. Importantly, at a median follow-up of 7.3 years, we showed that patients with OFR experienced a worse distant recurrence-free survival (DRFS) and overall survival (OS) when compared with patients without OFR [6].
The current study aims to update the prior results of the DATA study with six additional years of follow-up information. We first assess whether the long-term disease outcomes of patients who developed CIOFF remain similar to those of patients who were considered definitely postmenopausal. Subsequently, as the primary objective of this study, we compare the long-term disease outcomes of CIOFF patients who experienced OFR with those of CIOFF patients who did not experience OFR during treatment with anastrozole.
Methods
Study population
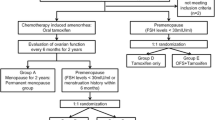
Patients were identified from the DATA study (NCT00301457): a phase 3, randomised controlled trial, which compared the efficacy of 6 versus 3 years of anastrozole in postmenopausal women with HR+ BC who were disease-free after two to three years of adjuvant treatment with tamoxifen [12, 13]. The final study population consisted of 1860 patients who were recruited from 79 hospitals in the Netherlands between 2006 and 2009.
For the current analysis, all patients aged 45–57 years at randomisation who received (neo)adjuvant chemotherapy were identified and categorised into two groups: (1) patients with CIOFF and (2) definitely postmenopausal patients [6, 11]. The following exclusion criteria were applied: the use of a gonadotropin-releasing hormone (GnRH) agonist before randomisation and the lack of postmenopausal oestradiol (E2) levels at randomisation.
Data collection and definitions
Patients were categorised as experiencing CIOFF when the last menstrual bleeding was reported within one year before the start of chemotherapy and postmenopausal E2 levels were present at randomisation. Patients were categorised as definitely postmenopausal when the last menstrual bleeding was reported more than one year before the start of chemotherapy or a bilateral ovariectomy was performed before randomisation.
In patients with CIOFF, E2 and follicle-stimulating hormone (FSH) levels were measured every six months during the first 30 months after randomisation to monitor for the incidence of OFR, which was defined by either a return of menstrual bleeding and/or the presence of premenopausal E2 and FSH levels. Local reference values from all participating hospitals were used to define pre- and postmenopausal E2 and FSH levels. In patients with OFR, treatment adjustments were based on the decision of the treating physician.
All study participants were monitored for disease recurrence and death every 6 months in the first 6 years after randomisation and yearly thereafter. A mammogram was performed once a year. Database lock: March 7, 2022.
Endpoints
The primary endpoint was disease-free survival (DFS), defined as time from randomisation until the occurrence of any of the following events: (non-)invasive BC recurrence, (non-)invasive second primary (breast) cancer other than basal cell or squamous cell carcinoma of the skin or carcinoma in situ of the cervix, or death from any cause. Secondary endpoints included DRFS, OS, BC-specific mortality (BCSM), and other-cause mortality (OCM). A period of DRFS ended following the development of a distant recurrence or death from any cause, whilst a period of OS ended following death from any cause. All BC-related deaths were included as events in the analysis of BCSM, whereas all non-BC-related deaths were included as events in the analysis of OCM.
Statistical analysis
Baseline characteristics of the study population were compared using the Chi-squared test for categorical variables and the Mann–Whitney U test for continuous variables.
Disease outcomes of patients with CIOFF were compared with those of definitely postmenopausal patients from randomisation onwards. Survival curves for DFS, DRFS, and OS were estimated with the Kaplan–Meier method. Mortality curves for BCSM and OCM were estimated with the cumulative incidence function, thereby considering non-BC-related death (BCSM) and BC-related death (OCM) as competing events. Differences between groups were examined with the log-rank test and the Gray’s test. Patients without an event were censored at the last follow-up date in all analyses.
In addition, multivariable Cox regression analyses were performed to evaluate whether patients with CIOFF experienced any differences in, respectively, DFS, DRFS, and OS when compared with definitely postmenopausal patients. The proportional hazards assumption was tested. The Fine and Gray method was used for BCSM and OCM, thereby considering, respectively, OCM and BCSM as competing events. Confounding factors included tumour size, nodal status, histological grade, and hormone receptor status. Age at last menstruation and body mass index (BMI) were excluded as confounding factors to avoid multicollinearity with postmenopausal status. Missing values of confounding factors were imputed.
Disease outcomes of patients with OFR were compared with those of patients without OFR, using the same statistical methods as described above. Multivariable analyses included OFR as a time-dependent covariate and excluded age as a confounding factor to avoid multicollinearity with OFR. In addition, for graphical presentation, a landmark analysis starting at 1 year after randomisation was performed to compare the disease outcomes of patients with OFR during the first year of treatment with those of patients without OFR during the first year of treatment. A landmark of one year was chosen, because the majority of OFR events occurred during the first year [11]. Patients who developed an endpoint event during the first year after randomisation were excluded from the landmark analysis.
All p values were two-sided and considered statistically significant at a value of ≤ 0.05. All statistical analyses were performed with SPSS (version 28), Stata (version 17), and RStudio (version 2023).
Results
In the current study, 656 patients were included: 395 patients with CIOFF and 261 patients who were considered definitely postmenopausal at randomisation (Fig. 1). Amongst the 395 patients with CIOFF, 329 patients had follow-up E2 and FSH measurements available to monitor for the incidence of OFR. These patients were considered eligible for the analysis assessing the prognostic impact of OFR. Overall, 39 patients experienced OFR, whereas 290 patients did not experience OFR during the first 30 months after randomisation. Menstrual bleeding was reported in 19 (49%) of 39 patients with OFR [6, 11]. Treatment adjustments were performed in 27 (69%) of 39 patients with OFR: 12 patients received additional treatment with a GnRH agonist, seven patients underwent a bilateral ovariectomy, six patients switched to tamoxifen, and two patients received monotherapy with a GnRH agonist [6, 11]. All patients with a BC recurrence received treatment adjustments [6].
Baseline characteristics
The age of patients with CIOFF and patients who were considered definitely postmenopausal was similar at randomisation (median age: 51 years) (Table 1). The age at last menstruation (median age: 45 versus 48 years) was however by definition lower in patients who were already definitely postmenopausal, whereas the BMI was higher (BMI of ≥ 25 kg/m2: 60% versus 49%) when compared with patients with CIOFF. Definitely postmenopausal patients were more frequently diagnosed with node-negative disease (36% versus 26%), histological grade 3 tumours (38% versus 26%), and tumours expressing only one hormone receptor (26% versus 18%) when compared with patients with CIOFF.
Amongst patients with CIOFF, baseline characteristics of patients with versus without OFR were comparable, except that patients with OFR were younger at randomisation (median age: 48 versus 51 years) and last menstruation (median age: 45 versus 48 years).
Association between CIOFF and disease outcomes in the total study population
The median follow-up time was 13.3 years (interquartile range (IQR) 12.5–14.0), during which 193 DFS events, 136 DRFS events, and 116 OS events were reported (Table 2, Supplementary Table 1).
The 13-year DFS rate of patients with CIOFF [73.1% (95% confidence interval (CI) 68.4–77.3)] was higher than the 13-year DFS rate of definitely postmenopausal patients [68.2% (95% CI 62.1–73.6)], but this difference was not statistically significant in the multivariable analysis (hazard ratio (HR) = 0.79; 95% CI 0.59–1.06; p = 0.12) (Fig. 2a, Table 2).
A higher 13-year DRFS rate was also observed in patients with CIOFF [81.1% (95% CI 76.8–84.7)] versus patients who were definitely postmenopausal [77.0% (95% CI 71.4–81.7)], but again results were not statistically significant after adjustment for potential confounding factors (HR = 0.79; 95% CI 0.56–1.12; p = 0.18) (Fig. 2b, Table 2).
The 13-year OS rates were, respectively, 85.0% (95% CI 81.0–88.2) and 80.9% (95% CI 75.5–85.2), with an adjusted HR of 0.73 (95% CI 0.50–1.06; p = 0.10) (Fig. 2c, Table 2). There was no difference in BCSM (subdistribution (s)HR = 0.93; 95% CI 0.56–1.54; p = 0.78) between patient groups (Fig. 3a, Table 2). However, a statistically significant difference in OCM (sHR = 0.48; 95% CI 0.26–0.88; p = 0.02) was observed (Fig. 3b; Table 2). In fact, the 13-year OCM rate was 4.8% (95% CI 2.9–7.3) in patients with CIOFF versus 8.6% (95% CI 5.6–12.5) in definitely postmenopausal patients (Fig. 3b).
Association between OFR and disease outcomes in patients with CIOFF
Patients with CIOFF who developed OFR experienced a non-statistically significant deterioration in DFS (adjusted HR = 1.54; 95% CI 0.85–2.81; p = 0.16), DRFS (adjusted HR = 1.51; 95% CI 0.73–3.11; p = 0.26), OS (adjusted HR = 1.64; 95% CI 0.75–3.55; p = 0.21), and BCSM (adjusted sHR = 1.98; 95% CI 0.84–4.63; p = 0.12) when compared with those who did not develop OFR (Table 2). The survival curves of patients with versus without OFR during the first year of treatment are presented as landmark analyses from one year after randomisation onwards (Fig. 4a–d). The respective 13-year residual survival rates (95% CI) were 65.0% (43.4–80.0) in patients with OFR versus 76.8% (71.5–81.3) in patients without OFR for DFS, 72.9% (51.4–86.1) versus 84.0% (79.2–87.7) for DRFS, and 76.9% (55.7–88.9) versus 87.6% (83.1–91.0) for OS (Fig. 4a–c). The 13-year residual BCSM rate was 19.2% (7.0–36.0) in patients with OFR versus 8.7% (5.7–12.4) in patients without OFR (Fig. 4d).
Disease-free survival (a), distant recurrence-free survival (b), overall survival (c), and breast cancer-specific mortality (d) in patients with chemotherapy-induced ovarian function failure (CIOFF) who experienced ovarian function recovery (OFR) and patients with CIOFF who did not experience OFR during the first year of treatment with anastrozole, from 1 year after randomisation onwards
Discussion
In this long-term follow-up analysis of 656 patients with HR + BC from the phase 3 DATA study on the extended use of anastrozole, we observed that definitely postmenopausal patients experienced worse outcomes when compared with patients with CIOFF. In-depth analysis showed that this trend towards a worse outcome was not caused by a difference in BCSM, but by a statistically significant difference in OCM. Furthermore, amongst patients with CIOFF, we observed that patients with OFR experienced an increased risk of BCSM when compared with patients without OFR.
Results of our study suggest that definitely postmenopausal patients experience worse DFS, DRFS, and OS when compared with patients with CIOFF. We believe that there are several potential explanations for these differences in outcomes. First, we observed that definitely postmenopausal patients experienced a higher risk of dying from non-BC-related causes. Actually, we revealed that the 13-year OCM rate of patients with CIOFF (4.8%) was half as high as the 13-year OCM rate of definitely postmenopausal patients (8.6%) (sHR = 0.48; 0.26–0.88; p = 0.02). These data are important, considering the fact that these women were rather young at randomisation (median age: 51 years). The number of non-BC deaths in our study is too small to perform any additional analyses, but it is known that a younger age at menopause is associated with an increased risk of cardiovascular- and bone-related events [14,15,16,17,18,19,20]. Second, differences in BMI should be taken into account. We found that definitely postmenopausal patients were more likely to be overweight or obese at randomisation. Earlier, we reported that overweight and obese patients from the DATA study experienced a significantly worse outcome when compared with normal weight patients, which is in line with the results of several meta-analyses on the adverse prognostic effect of overweight and obesity in patients with BC [21,22,23]. Maintaining a healthy BMI after becoming postmenopausal is thus important. Third, it is possible that misclassification of menopausal status occurred, as date of last menstruation was self-reported by study participants. We however assume good correlation rates between self-reported and measured dates of last menstruation in the first few years after menopause.
The current analysis shows that OFR during treatment with an aromatase inhibitor is associated with a worse prognosis, even though E2 and FSH levels were measured twice yearly and the majority of patients with OFR received treatment adjustments. It is furthermore important to realise that OFR occurred in a relatively old group of patients who had received chemotherapy more than two to three years earlier. In our study, patients with OFR experienced a clinically relevant decrease in DFS (HR = 1.54; 95% CI 0.85–2.81; p = 0.16), DRFS (HR = 1.51; 95% CI 0.73–3.11; p = 0.26), and OS (HR = 1.64; 95% CI 0.75–3.55; p = 0.21) when compared with patients without OFR during treatment with anastrozole. Patients with OFR also experienced a potentially meaningful increase in BCSM (sHR = 1.98; 95% CI 0.84–4.63; p = 0.12). In fact, the 13-year BCSM rate was twice as high in patients with OFR (19.2%) versus patients without OFR (8.7%). Up to this point, only one other prospective cohort study evaluated the association between OFR and disease outcomes in 53 HR + BC patients with CIOFF who received an aromatase inhibitor after initial treatment with tamoxifen [8]. In that study, an adverse association between OFR and DFS (HR = 9.3; 95% CI 3.3–48; p = 0.04) was also reported [8]. These findings do not come as a surprise, given the fact that aromatase inhibitors exert negative feedback on the hypothalamus–pituitary–ovary axis, stimulate gonadotropin secretion, and ultimately strongly increase ovarian oestrogen production in premenopausal women. These findings do however once again underscore the importance of OFS in patients with CIOFF who are considered potential candidates for treatment with an aromatase inhibitor. In fact, results of our study suggest that aromatase inhibitors cannot safely be prescribed without adequate OFS, i.e. additional treatment with a GnRH agonist or performance of a bilateral ovariectomy, in patients with CIOFF.
Long-term follow-up results of the SOFT trial showed that premenopausal women with HR + BC who received five years of tamoxifen in combination with 5 years of OFS experienced clinically relevant improvements in DFS (HR = 0.82; 95% CI 0.69–0.98; p = 0.03) and OS (HR = 0.78; 95% CI 0.60–1.01; p = 0.06) when compared with premenopausal women with HR + BC who received 5 years of tamoxifen monotherapy [24]. The absolute benefit of OFS differed between patient subgroups, showing the greatest benefit in patients who received chemotherapy and patients with “high-risk” clinicopathological features, such as age under 35 years or histological grade 3 tumours [24]. Results of the SOFT trial were corroborated by results of the ASTRRA trial, which showed that premenopausal women with HR + BC who had all been treated with chemotherapy experienced major improvements in DFS (HR = 0.67; 95% CI 0.51–0.87; p = 0.003), but not OS (HR = 0.78; 95% CI 0.49–1.25; p = 0.31), when receiving five years of tamoxifen in combination with 2 years of OFS versus 5 years of tamoxifen monotherapy [25]. The lack of OS benefit in the ASTRRA trial suggests that 5 years of OFS is superior to 2 years of OFS. A longer duration of OFS may however increase the risk of side effects which may reduce treatment compliance and quality of life. The most optimal duration of OFS is therefore still a matter of debate.
The incorporation of OFS has allowed researchers to explore the potential of aromatase inhibitors in the treatment of premenopausal women with HR + BC [26,27,28]. It is well known that aromatase inhibitors, either upfront or sequentially after two to three years of tamoxifen, are more effective than tamoxifen in the treatment of postmenopausal women with HR + BC [2]. A recent Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis has now shown that aromatase inhibitors are also more effective than tamoxifen in premenopausal women with oestrogen receptor-positive (ER +) BC who received OFS [29]. In fact, aromatase inhibitors reduced the relative risk of recurrence and distant recurrence by 21% and 17%, respectively [29]. So far, however, no difference in BCSM (rate ratio = 1.01; 95% CI 0.82–1.24; p = 0.94) has been observed [29]. The absolute benefit of treatment with an aromatase inhibitor versus tamoxifen in premenopausal women with HR + BC who received OFS is furthermore small, with a 10-year absolute benefit of, respectively, 2.8% for risk of recurrence and 1.9% for risk of distant recurrence [29]. The absolute benefit is expected to be even smaller in patients with a low risk of recurrence. Treatment with an aromatase inhibitor has also been associated with several side effects, such as musculoskeletal symptoms, osteoporosis, or vaginal dryness [28,29,30]. In patients with low-risk disease, aromatase inhibitors may therefore do more harm than good, especially considering the fact that 12-year OS rates of patients who did not receive chemotherapy exceeded 95% in all three treatment arms of the SOFT trial [24]. These data indicate that appropriate patient selection, considering risks and benefits of OFS, is important.
Our study is the largest study to date assessing the impact of OFR on the disease outcomes of HR + BC patients with CIOFF who received treatment with anastrozole after 2–3 years of tamoxifen. Major strengths of this study include the long-term follow-up period of currently more than 13 years beyond randomisation and the use of data from patients who participated in a randomised controlled trial, in which regular E2 and FSH measurements were performed and patients were consistently monitored during follow-up. This study also has some limitations. Several assays were used to define pre- and postmenopausal E2 and FSH levels across participating hospitals, thereby potentially influencing the incidence of OFR. Guerrero et al. however showed that the incidence of OFR was similar when using two different assays for determining OFR in the same patient [8]. Furthermore, the number of patients with OFR was low, which limited the performance of additional subgroup analyses and impacted the power of our results. A meta-analysis, including all studies on adjuvant endocrine therapy in HR + BC patients with CIOFF, could provide more information on the incidence, prognostic impact, and most optimal treatment of OFR.
In this long-term follow-up analysis, amongst a subset of patients with HR + BC from the phase 3 DATA study, we observed that patients with CIOFF who developed OFR during treatment with anastrozole experienced a potentially clinically relevant deterioration in BC outcomes when compared with patients who did not develop OFR. These findings underscore the importance of adequate OFS in patients with CIOFF who receive treatment with an aromatase inhibitor and support additional research on this topic. A meta-analysis assessing the prognostic impact of OFR in HR + BC patients with CIOFF receiving adjuvant aromatase inhibitor therapy is warranted.
Data availability
Data will be shared with interested researchers who are able to provide a methodologically sound proposal with well-defined research questions. Researchers are welcome to contact the corresponding author for more information at vcg.tjan.heijnen@mumc.nl.
Abbreviations
- BC:
-
Breast cancer
- BCSM:
-
Breast cancer-specific mortality
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CIOFF:
-
Chemotherapy-induced ovarian function failure
- DFS:
-
Disease-free survival
- DRFS:
-
Distant recurrence-free survival
- E2:
-
Oestradiol
- FSH:
-
Follicle-stimulating hormone
- GnRH:
-
Gonadotropin-releasing hormone
- HR:
-
Hazard ratio
- HR + :
-
Hormone receptor-positive
- IQR:
-
Interquartile range
- OCM:
-
Other-cause mortality
- OFR:
-
Ovarian function recovery
- OFS:
-
Ovarian function suppression
- OS:
-
Overall survival
- sHR:
-
Subdistribution hazard ratio
References
Loibl S, André F, Bachelot T, Barrios CH, Bergh J, Burstein HJ, Cardoso MJ, Carey LA, Dawood S, Del Mastro L, Denkert C, Fallenberg EM, Francis PA, Gamal-Eldin H, Gelmon K, Geyer CE, Gnant M, Guarneri V, Gupta S, Kim SB, Krug D, Martin M, Meattini I, Morrow M, Janni W, Paluch-Shimon S, Partridge A, Poortmans P, Pusztai L, Regan MM, Sparano J, Spanic T, Swain S, Tjulandin S, Toi M, Trapani D, Tutt A, Xu B, Curigliano G, Harbeck N (2024) Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up<sup>☆</sup>. Ann Oncol 35:159–182. https://doi.org/10.1016/j.annonc.2023.11.016
Early Breast Cancer Trialists' Collaborative Group (EBCTCG) (2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386:1341–1352. https://doi.org/10.1016/s0140-6736(15)61074-1
Pagani O, O’Neill A, Castiglione M, Gelber RD, Goldhirsch A, Rudenstam CM, Lindtner J, Collins J, Crivellari D, Coates A, Cavalli F, Thürlimann B, Simoncini E, Fey M, Price K, Senn HJ (1998) Prognostic impact of amenorrhoea after adjuvant chemotherapy in premenopausal breast cancer patients with axillary node involvement: results of the international Breast Cancer Study Group (IBCSG) trial VI. Eur J Cancer 34:632–640. https://doi.org/10.1016/S0959-8049(97)10036-3
Walshe JM, Denduluri N, Swain SM (2006) Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol 24:5769–5779. https://doi.org/10.1200/jco.2006.07.2793
Vriens IJ, De Bie AJ, Aarts MJ, de Boer M, van Hellemond IE, Roijen JH, van Golde RJ, Voogd AC, Tjan-Heijnen VC (2017) The correlation of age with chemotherapy-induced ovarian function failure in breast cancer patients. Oncotarget 8:11372–11379. https://doi.org/10.18632/oncotarget.14532
van Hellemond IEG, Vriens IJH, Peer PGM, Swinkels ACP, Smorenburg CH, Seynaeve CM, van der Sangen MJC, Kroep JR, de Graaf H, Honkoop AH, Erdkamp FLG, van den Berkmortel F, de Boer M, de Roos WK, Linn SC, Imholz ALT, Tjan-Heijnen VCG (2019) Efficacy of anastrozole after tamoxifen in early breast cancer patients with chemotherapy-induced ovarian function failure. Int J Cancer 145:274–283. https://doi.org/10.1002/ijc.32093
Smith IE, Dowsett M, Yap YS, Walsh G, Lønning PE, Santen RJ, Hayes D (2006) Adjuvant aromatase inhibitors for early breast cancer after chemotherapy-induced amenorrhoea: caution and suggested guidelines. J Clin Oncol 24:2444–2447. https://doi.org/10.1200/jco.2005.05.3694
Guerrero A, Gavilá J, Folkerd E, Ortiz B, Martínez F, García A, Climent MA, Guillem V, Ruíz A (2013) Incidence and predictors of ovarian function recovery (OFR) in breast cancer (BC) patients with chemotherapy-induced amenorrhea (CIA) who switched from tamoxifen to exemestane. Ann Oncol 24:674–679. https://doi.org/10.1093/annonc/mds464
Henry NL, Xia R, Banerjee M, Gersch C, McConnell D, Giacherio D, Schott AF, Pearlman M, Stearns V, Partridge AH, Hayes DF (2013) Predictors of recovery of ovarian function during aromatase inhibitor therapy. Ann Oncol 24:2011–2016. https://doi.org/10.1093/annonc/mdt149
Krekow LK, Hellerstedt BA, Collea RP, Papish S, Diggikar SM, Resta R, Vukelja SJ, Holmes FA, Reddy PK, Asmar L, Wang Y, Fox PS, Peck SR, O’Shaughnessy J (2016) Incidence and predictive factors for recovery of ovarian function in amenorrheic women in their 40s treated with letrozole. J Clin Oncol 34:1594–1600. https://doi.org/10.1200/jco.2015.62.2985
van Hellemond IEG, Vriens IJH, Peer PGM, Swinkels ACP, Smorenburg CH, Seynaeve CM, van der Sangen MJC, Kroep JR, de Graaf H, Honkoop AH, Erdkamp FLG, van den Berkmortel F, Kitzen J, de Boer M, de Roos WK, Linn SC, Imholz ALT, Tjan-Heijnen VCG (2017) Ovarian function recovery during anastrozole in breast cancer patients with chemotherapy-induced ovarian function failure. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djx074
Tjan-Heijnen VCG, van Hellemond IEG, Peer PGM, Swinkels ACP, Smorenburg CH, van der Sangen MJC, Kroep JR, De Graaf H, Honkoop AH, Erdkamp FLG, van den Berkmortel F, de Boer M, de Roos WK, Linn SC, Imholz ALT, Seynaeve CM (2017) Extended adjuvant aromatase inhibition after sequential endocrine therapy (DATA): a randomised, phase 3 trial. Lancet Oncol 18:1502–1511. https://doi.org/10.1016/s1470-2045(17)30600-9
Tjan-Heijnen VCG, Lammers SWM, Geurts SME, Vriens IJH, Swinkels ACP, Smorenburg CH, van der Sangen MJC, Kroep JR, de Graaf H, Honkoop AH, Erdkamp FLG, de Roos WK, Linn SC, Imholz ALT (2023) Extended adjuvant aromatase inhibition after sequential endocrine therapy in postmenopausal women with breast cancer: follow-up analysis of the randomised phase 3 DATA trial. eClinicalMedicine 58:101901. https://doi.org/10.1016/j.eclinm.2023.101901
Zhu D, Chung HF, Dobson AJ, Pandeya N, Giles GG, Bruinsma F, Brunner EJ, Kuh D, Hardy R, Avis NE, Gold EB, Derby CA, Matthews KA, Cade JE, Greenwood DC, Demakakos P, Brown DE, Sievert LL, Anderson D, Hayashi K, Lee JS, Mizunuma H, Tillin T, Simonsen MK, Adami HO, Weiderpass E, Mishra GD (2019) Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health 4:e553–e564. https://doi.org/10.1016/s2468-2667(19)30155-0
Muka T, Oliver-Williams C, Kunutsor S, Laven JSE, Fauser BCJM, Chowdhury R, Kavousi M, Franco OH (2016) Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiology 1:767–776. https://doi.org/10.1001/jamacardio.2016.2415
Sullivan SD, Lehman A, Thomas F, Johnson KC, Jackson R, Wactawski-Wende J, Ko M, Chen Z, Curb JD, Howard BV (2015) Effects of self-reported age at nonsurgical menopause on time to first fracture and bone mineral density in the Women’s Health Initiative Observational Study. Menopause 22:1035–1044. https://doi.org/10.1097/GME.0000000000000451
Paganini-Hill A, Atchison KA, Gornbein JA, Nattiv A, Service SK, White SC (2005) Menstrual and reproductive factors and fracture risk: the Leisure World Cohort Study. J Womens Health (Larchmt) 14:808–819. https://doi.org/10.1089/jwh.2005.14.808
Hadjidakis DJ, Kokkinakis EP, Sfakianakis ME, Raptis SA (2003) Bone density patterns after normal and premature menopause. Maturitas 44:279–286. https://doi.org/10.1016/s0378-5122(03)00040-9
Shieh A, Ruppert KM, Greendale GA, Lian Y, Cauley JA, Burnett-Bowie SA, Karvonen-Guttierez C, Karlamangla AS (2022) Associations of age at menopause with postmenopausal bone mineral density and fracture risk in women. J Clin Endocrinol Metab 107:e561–e569. https://doi.org/10.1210/clinem/dgab690
Anagnostis P, Siolos P, Gkekas NK, Kosmidou N, Artzouchaltzi AM, Christou K, Paschou SA, Potoupnis M, Kenanidis E, Tsiridis E, Lambrinoudaki I, Stevenson JC, Goulis DG (2019) Association between age at menopause and fracture risk: a systematic review and meta-analysis. Endocrine 63:213–224. https://doi.org/10.1007/s12020-018-1746-6
Lammers SWM, Geurts SME, van Hellemond IEG, Swinkels ACP, Smorenburg CH, van der Sangen MJC, Kroep JR, de Graaf H, Honkoop AH, Erdkamp FLG, de Roos WK, Linn SC, Imholz ALT, Smidt ML, Vriens IJH, Tjan-Heijnen VCG (2023) The prognostic and predictive effect of body mass index in hormone receptor-positive breast cancer. JNCI Cancer Spectr 7. https://doi.org/10.1093/jncics/pkad092
Chan DSM, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, Navarro Rosenblatt D, Thune I, Vieira R, Norat T (2014) Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 25:1901–1914. https://doi.org/10.1093/annonc/mdu042
Lohmann AE, Soldera SV, Pimentel I, Ribnikar D, Ennis M, Amir E, Goodwin PJ (2021) Association of obesity with breast cancer outcome in relation to cancer subtypes: a meta-analysis. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djab023
Francis PA, Fleming GF, Láng I, Ciruelos EM, Bonnefoi HR, Bellet M, Bernardo A, Climent MA, Martino S, Bermejo B, Burstein HJ, Davidson NE, Geyer CE, Walley BA, Ingle JN, Coleman RE, Müller B, Du FL, Loibl S, Winer EP, Ruepp B, Loi S, Colleoni M, Coates AS, Gelber RD, Goldhirsch A, Regan MM, Investigators ftS, Group tIBCS, Investigators ftS, Group tIBCS (2023) Adjuvant endocrine therapy in premenopausal breast cancer: 12-year results from SOFT. J Clin Oncol 41:1370–1375. https://doi.org/10.1200/jco.22.01065
Baek SY, Noh WC, Ahn SH, Kim HA, Ryu JM, Kim SI, Lee EG, Im SA, Jung Y, Park MH, Park KH, Kang SH, Jeong J, Park E, Kim SY, Lee MH, Kim LS, Lim W, Kim S, Kim HJ (2023) Adding ovarian suppression to tamoxifen for premenopausal women with hormone receptor-positive breast cancer after chemotherapy: an 8-year follow-up of the ASTRRA trial. J Clin Oncol 41:4864–4871. https://doi.org/10.1200/jco.23.00557
Pagani O, Walley BA, Fleming GF, Colleoni M, Láng I, Gomez HL, Tondini C, Burstein HJ, Goetz MP, Ciruelos EM, Stearns V, Bonnefoi HR, Martino S, Geyer CE Jr, Chini C, Puglisi F, Spazzapan S, Ruhstaller T, Winer EP, Ruepp B, Loi S, Coates AS, Gelber RD, Goldhirsch A, Regan MM, Francis PA (2023) Adjuvant exemestane with ovarian suppression in premenopausal breast cancer: long-term follow-up of the combined TEXT and SOFT trials. J Clin Oncol 41:1376–1382. https://doi.org/10.1200/jco.22.01064
Gnant M, Mlineritsch B, Stoeger H, Luschin-Ebengreuth G, Knauer M, Moik M, Jakesz R, Seifert M, Taucher S, Bjelic-Radisic V, Balic M, Eidtmann H, Eiermann W, Steger G, Kwasny W, Dubsky P, Selim U, Fitzal F, Hochreiner G, Wette V, Sevelda P, Ploner F, Bartsch R, Fesl C, Greil R (2015) Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann Oncol 26:313–320. https://doi.org/10.1093/annonc/mdu544
Perrone F, De Laurentiis M, De Placido S, Orditura M, Cinieri S, Riccardi F, Ribecco AS, Putzu C, Del Mastro L, Rossi E, Tinessa V, Mosconi AM, Nuzzo F, Di Rella F, Gravina A, Iodice G, Landi G, Pacilio C, Forestieri V, Lauria R, Fabbri A, Ibrahim T, De Maio E, Barni S, Gori S, Simeon V, Arenare L, Daniele G, Piccirillo MC, Normanno N, de Matteis A, Gallo C (2019) Adjuvant zoledronic acid and letrozole plus ovarian function suppression in premenopausal breast cancer: HOBOE phase 3 randomised trial. Eur J Cancer 118:178–186. https://doi.org/10.1016/j.ejca.2019.05.004
Bradley R, Braybrooke J, Gray R, Hills RK, Liu Z, Pan H, Peto R, Dodwell D, McGale P, Taylor C, Francis PA, Gnant M, Perrone F, Regan MM, Berry R, Boddington C, Clarke M, Davies C, Davies L, Duane F, Evans V, Gay J, Gettins L, Godwin J, James S, Liu H, MacKinnon E, Mannu G, McHugh T, Morris P, Read S, Straiton E, Jakesz R, Fesl C, Pagani O, Gelber R, De Laurentiis M, De Placido S, Gallo C, Albain K, Anderson S, Arriagada R, Bartlett J, Bergsten-Nordström E, Bliss J, Brain E, Carey L, Coleman R, Cuzick J, Davidson N, Del Mastro L, Di Leo A, Dignam J, Dowsett M, Ejlertsen B, Goetz M, Goodwin P, Halpin-Murphy P, Hayes D, Hill C, Jagsi R, Janni W, Loibl S, Mamounas EP, Martín M, Mukai H, Nekljudova V, Norton L, Ohashi Y, Pierce L, Poortmans P, Pritchard KI, Raina V, Rea D, Robertson J, Rutgers E, Spanic T, Sparano J, Steger G, Tang G, Toi M, Tutt A, Viale G, Wang X, Whelan T, Wilcken N, Wolmark N, Cameron D, Bergh J, Swain SM (2022) Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: a patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol 23:382–392. https://doi.org/10.1016/S1470-2045(21)00758-0
Francis PA, Pagani O, Fleming GF, Walley BA, Colleoni M, Láng I, Gómez HL, Tondini C, Ciruelos E, Burstein HJ, Bonnefoi HR, Bellet M, Martino S, Geyer CE, Goetz MP, Stearns V, Pinotti G, Puglisi F, Spazzapan S, Climent MA, Pavesi L, Ruhstaller T, Davidson NE, Coleman R, Debled M, Buchholz S, Ingle JN, Winer EP, Maibach R, Rabaglio-Poretti M, Ruepp B, Di Leo A, Coates AS, Gelber RD, Goldhirsch A, Regan MM (2018) Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med 379:122–137. https://doi.org/10.1056/NEJMoa1803164
Acknowledgements
The DATA study was funded by AstraZeneca. The funding source was involved in the design and monitoring of the DATA trial, but had no role in data collection, data analysis, data interpretation, and writing of the report. We would like to thank all treating physicians, trial site principal investigators, and local data managers who contributed to the recruitment, treatment, and follow-up of study participants.
Funding
This work was supported by AstraZeneca.
Author information
Authors and Affiliations
Consortia
Contributions
SWML, SMEG, IJHV, and VCGT-H contributed to conceptualisation, methodology, and investigation; ACPS, CHS, JRK, HdG, AHH, FWPJvdB, WKdR, ALTI, and VCGT-H contributed to resources; SWML, KEPEH, and SMEG performed data curation, formal analysis, and visualisation; VCGT-H performed supervision; SWML prepared the original draft of the manuscript. All authors carefully reviewed the first draft of the manuscript and provided feedback when necessary. SWML, SMEG, IJHV, and VCGT-H discussed feedback and prepared the final manuscript. All authors gave approval for publication of the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
SWML reports grants from AstraZeneca during the conduct of the study; grants from Eli Lilly outside the submitted work. SWML received speaker honoraria from Novartis outside the submitted work. SMEG reports grants from AstraZeneca during the conduct of the study; institutional grants from Roche, Pfizer, Novartis, Eli Lilly, Daiichi Sankyo, AstraZeneca, and Gilead outside the submitted work and personal fees from AstraZeneca outside the submitted work. ACPS and ALTI report grants from AstraZeneca during the conduct of the study. JRK reports grants from AstraZeneca, MSD, Eisai, Eli Lilly, and GSK outside the submitted work. AHH reports grants from the Dutch Breast Cancer Research Group during the conduct of the study and outside the submitted work. AHH has been an Advisory Board member for Eli Lilly. AHH received support from Pfizer to attend the ESMO 2022 congress. IJHV reports grants from AstraZeneca during the conduct of the study and grants from Pfizer and Eli Lilly outside the submitted work. VCGT-H reports grants and personal fees from AstraZeneca during the conduct of the study and outside the submitted work; grants and personal fees from Novartis and Eli Lilly; and grants from Roche, Pfizer, Daiichi Sankyo, and Gilead outside the submitted work. VCGT-H has a consulting role for AstraZeneca, Eli Lilly, and Novartis. The other authors have declared no conflicts of interest.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the medical ethics committee of the Radboud University Medical Centre, Nijmegen.
Consent to participate
All patients provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding author during the submission and pre-publication phase: Senna W.M. Lammers.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lammers, S.W.M., Geurts, S.M.E., Hermans, K.E.P.E. et al. Ovarian function recovery in breast cancer patients receiving adjuvant anastrozole treatment: updated results from the phase 3 DATA trial. Breast Cancer Res Treat (2024). https://doi.org/10.1007/s10549-024-07411-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10549-024-07411-w