Abstract
Purpose
Breast cancer is the most frequent cancer in women with significant death rate. Morbidity is associated with drug resistance and metastasis. Development of novel drugs is unmet need. The aim of this study is to show potent anti-neoplastic activity of the UM171 compound on breast cancer cells and its mechanism of action.
Methods
The inhibitory effect of UM171 on several breast cancer (BC) cell lines was examined using MTT and colony-forming assays. Cell cycle and apoptosis assays were utilized to determine the effect of UM171 on BC cell proliferation and survival. Wound healing scratch and transwell migration assays were used to examine the migration of BC cell lines in culture. Xenograft of mouse model with 4T1 cells was used to determine inhibitory effect of UM171 in vivo. Q-RT-PCR and western blotting were used to determine the expression level of genes effected by UM171. Lentivirus-mediated shRNAs were used to knockdown the expression of KLF2 in BC cells.
Results
UM171 was previously identified as a potent agonist of human hematopoietic stem cell renewal and inhibitor of leukemia. In this study, UM171 was shown to inhibit the growth of multiple breast cancer cell lines in culture. UM171-mediated growth inhibition was associated with the induction of apoptosis, G2/M cell cycle arrest, lower colony-forming capacity, and reduced motility. In a xenotransplantation model of mouse triple-negative breast cancer 4T1 cells injected into syngeneic BALB/c mice, UM171 strongly inhibited tumor growth at a level comparable to control paclitaxel. UM171 increased the expression of the three PIM genes (PIM1-3) in breast cancer cells. Moreover, UM171 strongly induced the expression of the tumor suppressor gene KLF2 and cell cycle inhibitor P21CIP1. Accordingly, knockdown of KLF2 using lentivirus-mediated shRNA significantly attenuated the growth suppressor activity of UM171. As PIM1-3 act as oncogenes and are involved in breast cancer progression, induction of these kinases likely impedes the inhibitory effect of KLF2 induction by UM171. Accordingly, combination of UM171 with a PAN-PIM inhibitor LGH447 significantly reduced tumor growth in culture.
Conclusion
These results suggested that UM171 inhibited breast cancer progression in part through activation of KLF2 and P21. Combination of UM171 with a PAN-PIM inhibitor offer a novel therapy for aggressive forms of breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With substantial advances in diagnosis and treatment, breast cancer (BC) is still a major cause of mortality in women. Despite conventional therapies, patients succumb to the disease due to metastasis, drug resistance, and tumor recurrence [1, 2]. Therefore, development of new drug targeting breast cancer cells is critical for eradication of this disease.
The pyrimido-indole derivative UM171 was previously developed to expand hematopoietic stem cells in mouse [3] and human [4]. UM171 induces expression of endothelial protein C receptor (EPCR/CD201/PROCR) in a small subset of human cord blood CD34 + cells that may be responsible for HSC expansion [5]. Another study demonstrated that knockdown of the Lysine-Specific histone demethylase 1A (LSD1/KDM1) in mice expands HSCs similar to the phenotype seen in UM171-treated mice [6], suggesting another possible target of UM171. UM171 was later shown to potentiate the activity of a CULLIN3-E3 ubiquitin ligase (CRL3) complex to degrade Kelch/BTB domain protein KBTBD4. UM171 activation of the CULLIN3-KBTBD4 ubiquitin ligase then specifically targets LSD1 corepressor complex for proteasomal degradation, inducing HSC expansion [7]. In a recent study, UM171 was shown by us to have a strong anti-leukemia activity [8]. In addition to LSD1, we identified proto-oncogene serine/threonine-protein kinase PIM1 as another target of UM171. Binding of UM171 to PIM1 activates this kinase resulting in HSC expansion [8]. Interestingly, anti-leukemic activity of UM171 is independent of LSD1 and PIM1 [8] and is likely mediated through another target. The anti-leukemic activity of UM171 is attributed to its upregulation of the tumor suppressor genes KLF2 and P21CIP1 [8].
Cancer stem cells (CSCs) represent a subpopulation of tumor cells with different responses to cancer therapy that may underlie relapse [9]. The HSC expansion induced by UM171 was shown to interfere with its independent anti-leukemia activity in a mouse model of leukemia [8]. Therefore, combining UM171 with a PAN-PIM inhibitor resulted in better inhibitory effect in leukemia [8]. To combat Leukemia stem cells (LSCs), many inhibitors of LSD1 were developed for treatment of this malignancy [10]. The combination of LSD1 inhibitors with other anti-cancer drugs could eliminate relapse and provide a better therapy [11]. Combining LSD1 with UM171 is then expected to significantly improve cancer therapy [8].
KLF2 is one of the 18 member of the Krüppel-like transcription factors containing highly conserved DNA-binding zinc finger domains. In addition to various biological functions, KLF2 has been shown to be involved in progression of various cancers [12,13,14,15,16,17]. In a previous study, UM171 compound was shown to strongly induce the expression of KLF2 in erythroleukemia cells and ablation of its expression significantly attenuated growth suppression by this compound. This growth inhibition by KLF2 in leukemic cells was shown to be mediated independence of its stem cell expansion activity [8]. These results were further supported by the tumor suppressor function of KLF2 in various cancers.
Herein, we investigated the effect of UM171 on breast cancer. We showed that UM171 exerts a strong anti-neoplastic activity on breast cancer cell lines in culture and in a xenotransplantation mouse model. UM171 triggered upregulation of the PIM1-3 as well as KLF2, which in part mediated the anti-neoplastic activity of this compound. These results implicated UM171 as candidate drug for treatment of breast cancer and possibly other types of epithelial malignancies.
Materials and methods
Cells culture and compound treatment
The human and murine cell lines originated from breast cancer (MDA-MB-231, MCF-7 and 4T1), erythroleukemia (HEL), embryonic kidney (HEK293T) were all obtained from ATCC (US) and cultured as well as maintained in Dulbecco’s Modified Eagle Medium supplemented with 5% fetal bovine serum (HyClone, GE Healthcare, US).
For drug therapy, MDA-MB-231 cells (5 × 103), 4T1 cells (5 × 103), or MCF-7 (5 × 103) cells were seeded on 96-well plates and then treated with the indicated concentrations of UM171 or DMSO as the vehicle control. After 0 h, 24 h, 48 h or 72 h, cells were incubated with MTT (5 mg/mL) reagent (Solarbio 298-93-1, CN) for 4 h. Next, cells were incubated with dissolved solution (10% SDS, 5% isobutanol, and 0.012 mol/L HCL) overnight. Finally, absorbance was subsequently measured at 570 nm using a spectrophotometer (Gene Company Limited, CN) and a proliferation index was constructed via MTT assay. The PAN-PIM inhibitor (LGH447) was purchased from APE-BIO (A89505, US).
Cell cycle and apoptosis analyses
MDA-MB-231 cells (2 × 105) or 4T1 cells (2 × 105) were seeded on 6-well plates and then treated with the indicated concentrations of UM171 or DMSO as the vehicle control for 24 h. The morphology of cells was observed by inverted microscope (LEICA DMi8, Germany). For cell cycle analysis, obtained cells were fixed with 70% ethanol overnight at − 20 °C and then incubated with cycle staining buffer (PI: RNase A: TritonX-100 = 75:7.5:1) for 30 min at 37 °C. Cells were filtered using 200-mesh cell sieves and analyzed by flow cytometer (NovoCyte flow cytometer (ACEC Biosciences Inc., CA, US). For apoptosis analysis, cells were incubated with PI and Annexin V (BD FITC Annexin V Apoptosis Detection Kit I, #556547) for 15 min. Finally, cells were analyzed by flow cytometer using NovoCyte flow cytometer (ACEC Biosciences Inc., CA, US).
Wound healing scratch assay
For wound healing scratch assay, MDA-MB-231 cells (2 × 105) or 4T1 cells (2 × 105) were seeded on 6-well plates. The next day, scratches were made by a sterile 200 μL pipette tip and cells were treated with indicated drugs at the same time. Pictures were taken using an inverted microscope at 0 h, 24 h, and 48 h. Then, the area of the scratches was analyzed and used to calculate the wound healing ratio (%).
Transwell migration and clone-forming assay
For transwell migration, after treated with indicated drugs, cells (2 × 104) were inoculated in the upper chamber, and complete medium was added in the lower chamber. After incubated for 24 h at 37 °C, cotton swabs were used to remove the cells at the upper surface of membrane. After fixing with 4% paraformaldehyde, cells were stained by crystal violet reagent (Beyotime Biotechnology, #C0121, CN) and observed by inverted microscope. For clone-forming assay, after treated with indicated drugs, cells (1 × 103) were seeded on 6-well plates and cultured in complete medium for 14 days. After fixing with 4% paraformaldehyde, cells were stained by crystal violet reagent and observed by camera. Three discontinuous areas were used to analyze the counts of migration or clone forming.
RNA extraction and Q-RT-PCR
Cells (2 × 106) were seeded in 100 mm2 wells and treated with indicated drugs for 24 h. As described in previous study, we used the same procedures to extract RNA using TRIzol regent (Thermo Fisher Scientific, US) [8]. Then, RNA is reverse-transcribed into cDNA with the PrimeScript RT reagent kit (Takara Bio, CN) [8]. The Step One Plus Real-time PCR system (Thermo Fisher Scientific, US) was used for Q-RT-PCR as described previously [8], and the house-keeping gene GAPDH was set as a normalized control (100%). The primer sequences are listed in Table 1. All Q-RT-PCR experiments performed in three independent replicates for at least three biological replicates, each in triplicate (n = 3).
Western blot analysis
Cells (1 × 106) were seeded in 100 mm2 wells and treated with indicated drugs for 24 h. The proteins were then extracted using IP lysis for 30 min and used to detect by western blot. The bands were incubated with primary antibodies overnight at 4 °C and incubated with second antibodies for 2 h at room temperature. Finally, we used the Odyssey system (LI-COR Biosciences) to image and analyze the proteins, as described [18]. The antibodies are listed as follows: the GAPDH (AB-P-R001) antibody was obtained from Goodhere Biotech (CN); KLF2 antibody (222842) was obtained from ZEN-BIOSCIENCE (CN); goat anti-rabbit IgG (H + L) DyLight (TM) 800 (5151) and Anti-mouse IgG (H + L) DyLight (TM) 680 (5470) were obtained from Cell Signaling Technology (US).
ShRNA lentiviral construction
ShKLF2 Vigene Bioscience (Shangdong, CN) and scrambled control plasmids were cloned into the unique BcuI sites of the PLent-GFP expression vector (Vigene Bioscience, US). Using Lipofectamine 2000 (11668–019, Thermo Fisher Scientific, US), shKLF2 (6 μg) was co-transfected with packaging plasmids psPAX2 (3 μg) and pMD2.G (6 μg) (Addgene plasmid #12259 and #12260) into HEK293T cells for producing shRNA lentiviruses. After 48-h post-transfection, MDA-MB-231 cells were transduced with the supernatants, which were harvested and filtered through 0.45 µm filters. After 48 h, puromycin-resistant cells were selected by adding 5 mg/mL puromycin (Solarbio, Beijing, CN) in the medium. The sequences of shRNA are listed in Table 2.
4T1 breast cancer model and drug therapy
BALB/c females (5–6 weeks old) were purchased from Tengxin (SCXK2019-0010) (CN). After acclimation with the environment for a week, each mouse was implanted with 1 × 106 4T1 cells (100 μL of cell suspension) on the breast pat. At one week after cell inoculation, mice were intraperitoneally injected with UM171 (10 mg/Kg body weight) every other day for 7 times and paclitaxel (8 mg/Kg body weight) (MCE, HY-B0015, US) as the positive control. After a week of treatment, tumor volume (mm3) and body weight (g) were recorded for 28 days, when the tumor become smaller than the permitted size. The tumors were collected after mice were anesthetized with pentobarbital.
Statistical analysis
The statistical analysis was conducted using a two-tailed Student t test or a one-way ANOVA with Tukey’s post hoc test, using Graphpad Prism software. The threshold for significance was indicated within the figures as follows: * (P = < 0.05), ** (P = < 0.001), *** (P = < 0.0001), and **** (P = < 0.00001). The results were expressed as mean ± the standard deviation from at least 3 independent experiments.
Results
UM171 inhibited growth of breast cancer cell lines in culture
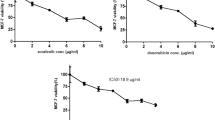
UM171 was previously shown to expand stemness and inhibit the growth of leukemic cells in culture and in vivo [8]. To study its effect on other types of cancer, we investigated the effect of UM171 on growth of several breast cancer cell lines. We found that UM171 effectively inhibited the proliferation of human MDA-MB-231 triple-negative (Fig. 1a) and MCF-7 luminal (Fig. 1b) as well as murine basal-like 4T1 (Fig. 1c) breast cancer cell lines in a dose-dependent manner. UM171 suppressed MDA-MB-231 proliferation with an IC50 of 2.1, while MCF-7 and 4T1 cells exhibited IC50 of 2.3 and 4, respectively (Fig. 1d). UM171 also strongly inhibited colony formation in a dose-dependent manner (Fig. 1e).
UM171 inhibited breast cancer cell proliferation in culture (a–c) Proliferation analysis of MDA-MB-231 (a), MCF-7 (b), and 4T1 (c) cells using the indicated doses of UM171, as determined by MTT assay. d The IC50 analysis of the indicated cell lines after treatment for 3 days with UM171. e MDA-MB-231 cells were treated with the indicated doses of UM171 for 24 h and plated for 14 days. Cells were then stained with crystal violet reagent and colonies were counted. Average of three independent experiments were shown below. P < 0.05 (*), P < 0.01 (**), P < 0.001 (***), and P < 0.0001 (****)
UM171 altered cell cycle progression and promoted apoptosis of MDA-MB-231 and 4T1 cells in culture
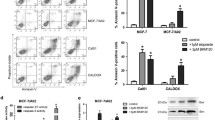
UM171 induced dose-dependent cell death in both MDA-MB-231 and 4T1 cells, as appeared by microscopic examination (Fig. 2a, b) and Annexin V-PI flow cytometry apoptotic assays (Fig. 2c, d, supplemental Figs. 1a and 2a). Cell cycle analysis of MDA-MB-231 cells revealed that UM171 reduced the percentage of cells in G1, with concomitant increased in cells in the G2/M phase of the cell cycle (Fig. 2e and supplemental Fig. 1b). In contrast, UM171 caused no significant effect on cell cycle parameters of 4T1 cells (Fig. 2f and supplemental Fig. 2b).
UM171 induced apoptosis and cell cycle arrest of breast cancer cells in culture (a, b) Microscopic images of MDA-MB-231 (a) and 4T1 (b) after treatment for 24 h with the indicated concentration of UM171 in culture. c, d The apoptosis index of MDA-MB-231 (c) and 4T1 (d) after treatment for 24 h with the indicated concentration of UM171. Average of three experiments were shown. e, f The cell cycle analysis of MDA-MB-231 (e) and 4T1 (f) cells after treatment with the indicated doses of UM171 for 24 h. Average of three experiments were shown. P < 0.05 (*), P < 0.01 (**), P < 0.001 (***), and P < 0.0001 (****)
UM171 inhibited migration of breast cancer cells in culture
To determine the effect of UM171 on cell migration, we next performed wound healing assay of MDA-MB-231 cells. UM171 significantly inhibited gap filling of the MDA-MB-231 cells in culture (Fig. 3a). Similar trend was also observed in tumor migration assay of MDA-MB-231 cells over 24 h in culture (Fig. 3b). A similar inhibition of tumor migration by UM171 was also observed in 4T1 cells (Fig. 4a, b). Together, these results revealed strong inhibition of both cell proliferation and migration by UM171 in breast cancer cells in culture.
UM171 blocked migration of MDA-MB-231 cells (a) UM171 in a dose-dependent manner inhibited gap filling of the MDA-MB-231 cells for the indicated times in culture (Left panel). Average of three experiments for 24 h and 48 h is shown in the right panel. b Tumor migration assay of MDA-MB-231 cells over 24 h after staining with crystal violet reagent (Left panel). Average of three independent experiments was shown in the right panel. P < 0.05 (*), P < 0.01 (**), P < 0.001 (***), and P < 0.0001 (****)
UM171 blocked migration of 4T1 cells (a) UM171 in a dose-dependent manner inhibited gap filling of the 4T1 cells for the indicated times in culture (Left panel). Average of three experiments for 24 h and 48 h is shown in the right panel. b Tumor migration assay of 4T1 cells over 24 h after staining with crystal violet reagent (Left panel). Average of three independent experiments is shown in the right panel. P < 0.01 (**), P < 0.001 (***), and P < 0.0001 (****)
UM171 suppressed growth of 4T1 breast cancer cells in vivo
Due to strong inhibitory effect of UM171 of several breast cancer cell lines in culture (Figs. 1, 2, 3, 4), we examined the ability of this compound to attenuate the growth of murine 4T1 cells in xenotransplantation model in vivo. Groups of BALB/c mice were injected into the mammary fat pads with 4T1 cells (1 × 106) and a week later were treated with UM171 (10 mg/kg) for 2 weeks (every other day). In this experiment, paclitaxel (8 mg/kg), commonly used for treatment of breast cancer [19], was used as positive control. Treatment with either UM171 or paclitaxel significantly inhibited growth of 4T1 in vivo (Fig. 5a–c). Whereas paclitaxel reduced body weight, albeit insignificantly, UM171 treatment did not significantly affect body weight, suggesting no overt side effects at this dose (Fig. 5d).
UM171 inhibited the progression of breast cancer 4T1 cells in vivo (a) BALB/c mice (n-5) were injected into the breast pads with 4T1 cells (1.0 × 106). Seven days after 4T1 injection, mice were treated with UM171 (10 mg/kg) for 2 weeks (every other day). In this experiment, paclitaxel (8 mg/kg) was used as control. Tumors were removed at 28 day post-cell injection and photographed. b Tumor weight of control and treated groups. (C-D) The tumor volume (c) and body weight (d) of the control and injected mice at the indicated time period. P < 0.01 (**), P < 0.001 (***), and P < 0.0001 (****)
UM171 induced the marker of breast cancer stemness and tumor suppressor genes KLF2 and P21CIP1
We previously showed that UM171 induced leukemia stem cell markers through binding to PIM18. This binding caused activation and higher expression of PIM1 [8]. Higher expression of PIM1 was also seen in MDA-MB-231 cells treated with UM171 (Fig. 6a). However, much higher level of induction was seen in HEL erythroleukemic cells treated with UM171, as previously reported (Fig. 6b) [8]. Interestingly, UM171 also induced PIM2 and PIM3 expression, indicating activation of all three PIM genes by this agent (Fig. 6c, d). As PIM1-3 kinases are implicated in breast cancer progression [20,21,22], we examined the combined effect of UM171 and PAN-PIM inhibitor LGH447 on proliferation of MDA-MB-231 cells. While LGH447 alone significantly inhibited cell proliferation, combined treatment with UM171 + LGH447 further reduced cell growth in culture (Fig. 6e).
Induction of the PIM1-3 oncogenes and growth suppressor KLF2/P21 genes by UM171 in breast cancer cells (a–d) Expression of PIM1 (a), PIM2 (c), and PIM3 (d) in MDA-MD-231 and PIM1 in HEL (b) cells after treatment with UM171 for 24 h, as determined by Q-RT-PCR. e Growth rate of MDA-MB-231 cells treated with UM171 (2 μM), LGH447 (5 μM), and UM171 + LGH447, for the indicated time period. f, g The expression of KLF2 (f) and P21 (g) after treatment with UM171 for 24 h, as determined by Q-RT-PCR. P < 0.05 (*), P < 0.01 (**), P < 0.001 (***), and P < 0.0001 (****)
Induction of the tumor suppressor KLF2 and the cell cycle inhibitor P21CIP1 by UM171 independently of PIM1 has previously been shown to be responsible in part for its anti-leukemic activity [8]. Transcription of both KLF2 and P21 genes was induced in MDA-MB-231 cells treated with UM171 (Fig. 6f, g).
UM171 inhibited cell proliferation in part through KLF2 induction
As KLF2 expression was significantly induced in MDA-MB-231 cells (Fig. 6f), we examined whether growth inhibitory effect of UM171 was mediated through this tumor suppressor gene. To this end, we knocked down KLF2 expression in MDA-MB-231 cells using lentivirus shRNA vectors. Expression of KLF2 was indeed significantly reduced in KLF2-sh1-, KLF2-sh2-, and KLF2-sh3-treated cells (Fig. 7a, b). KLF2 was robustly induced in control scrambled transfected cells, and was much reduced in KLF2-sh1 (Fig. 7c), KLF2-sh2 (Fig. 7d) and KLF2-sh3 (Fig. 7e) knockdown cells. Knockdown of KLF2 in MDA-MB-231 cells slightly, but significantly increased cell proliferation in KLF2-sh1 (Fig. 7f), KLF2-sh2 (Fig. 7g) and KLF2-sh3 (Fig. 7h) cells compared with control (NC-control) cells. When KLF2 knockdown cells were treated with UM171, the inhibitory rate of cell proliferation was significantly attenuated in KLF2-sh1 (Fig. 7f), KLF2-sh2 (Fig. 7g), and KLF2-sh3 (Fig. 7h) cells. These results suggested that activation of KLF2 by UM171 mediated in part its growth inhibition.
UM171 inhibited proliferation of breast cancer cells in part through the induction of KLF2 (a, b). The expression of KLF2 in the lentivirus MD-MB-231 infected KLF2-sh1, KLF2-sh2, KLF2-sh3, and scrambled control cells, as determined by Q-RT-PCR (a) or western blotting (b). c–e The expression of KLF2 in KLF2-sh1 (c), KLF2-sh2 (d), and KLF2-sh3 (e) and their scrambled control group after treatment with DMSO or UM171 (2 μM), as determined by Q-RT-PCR. f, h The growth rate of KLF2-sh1 (f), KLF2-sh2 (g), KLF2-sh3 (h), and their corresponding control cells treated with or without UM171 (2 μM). P < 0.01 (**), P < 0.001 (***), and P < 0.0001 (****)
Discussion
The compound UM171 was previously shown to inhibit growth of leukemia cells in culture and in vivo through alteration in expression of specific genes [8]. In this study, we showed that UM171 could also inhibit the growth and migration of breast cancer cell lines in culture as well as the progression of mouse 4T1 breast cancer xenografts in vivo. The in vivo suppressor activity of UM171 was similar to the effect seen by paclitaxel, a potent drug commonly used for treatment of breast cancer [19]. UM171 blocked the proliferation of triple-negative breast cancer cell lines MDA-MB-231 and 4T1, suggesting an important therapeutic value for this pyrimido-indole derivative compound for treatment of this disease and likely other types of cancer.
Similar to leukemic cells, UM171 inhibited proliferation, induced apoptosis, and cell cycle arrest in breast cancer cells. While UM171 induced G2/M cell cycle arrest in MDA-MB-231 cells, no effect on cell cycle was seen when 4T1 cells were treated with this compound, suggesting different effects on cell cycle progression. In addition, UM171 strongly inhibited colony-forming ability and migration potential of breast cancer cells. These functions likely underline the strong suppression of 4T1 tumors in mice by UM171 and further implicate this compound as a candidate for the treatment of aggressive forms of breast cancer.
UM171 was previously identified as an agent which at low doses (nM) increases expansion of hematopoietic stem cells [3]. In breast cancer cell lines, UM171 was shown to increase the expression of the oncogenes PIM1-3, which are involved in the progression of breast cancer [20,21,22]. Thus, in addition to breast cancer inhibitory activity, UM171 also induces an oncogenic effect that should imped its anti-neoplastic activity. In the past decade, the PIM (1–3) genes were identified as targets for breast and other cancers, and accordingly, many PAN-PIM inhibitors were developed to combine with other cancer therapies [23,24,25,26]. Indeed, combining UM171 with PAN-PIM inhibitor LGH447 further inhibited cancer cell growth. Thus, combining UM171 with a PAN-PIM kinase inhibitors should provide an excellent therapy for breast and likely other cancer types.
In breast cancer cell lines, UM171 was shown to activate the expression of tumor suppressor gene KLF2 [13, 27] and cell cycle inhibitor P21 [28]. Indeed, KLF2 was previously implicated as a tumor suppressor gene in breast cancer [29]. Accordingly, lentivirus-mediated knockdown of KLF2 in breast cancer cells significantly reduced growth suppression by UM171. These results implicated KLF2 as a potential target of UM171 that in part mediated growth suppression by this compound. As in leukemia, we have shown KLF2 induction independent of the PIM kinase pathway [8]. Further studies are needed to uncover the mechanism of induction of this tumor suppressor gene.
In conclusion, we have shown that UM171 exhibits a strong inhibitory activity in breast cancer cell lines associated with cell cycle arrest, apoptosis, lower migration, and colony-forming activities. This inhibitory activity of UM171 was also shown in xenografts of 4T1 breast cancer mouse model. While breast cancer inhibitory effect was in part associated with upregulation of P21CIP1 and KLF2, this compound also activated the expression of oncogenes PIM1-PIM3 that likely imped the anti-neoplastic of the compound. Therefore, combining UM171 with a PAN-PIM inhibitor could be used as a powerful tool for the treatment of triple-negative breast cancer and likely other type of malignancies.
Data availability
Data available upon request.
Abbreviations
- BC:
-
Breast cancer
- EPCR/CD201/PROCR:
-
Endothelial protein C receptor
- LSD1/KDM1:
-
Lysine-specific histone demethylase 1A
- CRL3:
-
CULLIN3-E3 ubiquitin ligase
- CSCs:
-
Cancer stem cells
- LSCs:
-
Leukemia stem cells
References
Phi LTH, Sari IN, Yang YG, Lee SH, Jun N, Kim KS, Lee YK, Kwon HY (2018) Cancer stem cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cells Int. https://doi.org/10.1155/2018/5416923
Sampieri K, Fodde R (2012) Cancer stem cells and metastasis. Semin Cancer Biol 22(3):187–193. https://doi.org/10.1016/j.semcancer.2012.03.002
Fares I, Chagraoui J, Gareau Y, Gingras S, Ruel R, Mayotte N, Csaszar E, Knapp DJ, Miller P, Ngom M, Imren S, Roy DC, Watts KL, Kiem HP, Herrington R, Iscove NN, Humphries RK, Eaves CJ, Cohen S, Marinier A, Zandstra PW, Sauvageau G (2014) Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science 345(6203):1509–1512. https://doi.org/10.1126/science.1256337
Cohen S, Roy J, Lachance S, Delisle JS, Marinier A, Busque L, Roy DC, Barabé F, Ahmad I, Bambace N, Bernard L, Kiss T, Bouchard P, Caudrelier P, Landais S, Larochelle F, Chagraoui J, Lehnertz B, Corneau S, Tomellini E, van Kampen JJA, Cornelissen JJ, Dumont-Lagacé M, Tanguay M, Li Q, Lemieux S, Zandstra PW, Sauvageau G (2020) Hematopoietic stem cell transplantation using single UM171-expanded cord blood: a single-arm, phase 1–2 safety and feasibility study. Lancet Haematol 7(2):e134–e145. https://doi.org/10.1016/s2352-3026(19)30202-9
Fares I, Chagraoui J, Lehnertz B, MacRae T, Mayotte N, Tomellini E, Aubert L, Roux PP, Sauvageau G (2017) EPCR expression marks UM171-expanded CD34(+) cord blood stem cells. Blood 129(25):3344–3351. https://doi.org/10.1182/blood-2016-11-750729
Subramaniam A, Žemaitis K, Talkhoncheh MS, Yudovich D, Bäckström A, Debnath S, Chen J, Jain MV, Galeev R, Gaetani M, Zubarev RA, Larsson J (2020) Lysine-specific demethylase 1A restricts ex vivo propagation of human HSCs and is a target of UM171. Blood 136(19):2151–2161. https://doi.org/10.1182/blood.2020005827
Chagraoui J, Girard S, Spinella JF, Simon L, Bonneil E, Mayotte N, MacRae T, Coulombe-Huntington J, Bertomeu T, Moison C, Tomellini E, Thibault P, Tyers M, Marinier A, Sauvageau G (2021) UM171 preserves epigenetic marks that are reduced in ex vivo culture of human HSCs via potentiation of the CLR3-KBTBD4 complex. Cell Stem Cell 28(1):48–62. https://doi.org/10.1016/j.stem.2020.12.002
Hu A, Gao J, Varier KM, Gajendran B, Jiang F, Liu W, Wang C, Xiao X, Li Y, Zacksenhaus E, Ali S, Ben-David Y (2022) UM171 cooperates with PIM1 inhibitors to restrict HSC expansion markers and suppress leukemia progression. Cell Death Discov 8(1):448. https://doi.org/10.1038/s41420-022-01244-6
Wang JC, Dick JE (2005) Cancer stem cells: lessons from leukemia. Trends Cell Biol 15(9):494–501. https://doi.org/10.1016/j.tcb.2005.07.004
Agboyibor C, Dong J, Effah CY, Drokow EK, Pervaiz W, Liu HM (2021) LSD1 as a biomarker and the outcome of its inhibitors in the clinical trial: the therapy opportunity in tumor. J Oncol. https://doi.org/10.1155/2021/5512524
Ravasio R, Ceccacci E, Nicosia L, Hosseini A, Rossi PL, Barozzi I, Fornasari L, Zuffo RD, Valente S, Fioravanti R, Mercurio C, Varasi M, Mattevi A, Mai A, Pavesi G, Bonaldi T, Minucci S (2020) Targeting the scaffolding role of LSD1 (KDM1A) poises acute myeloid leukemia cells for retinoic acid-induced differentiation. Sci Adv 6(15):2746. https://doi.org/10.1126/sciadv.aax2746
Wu N, Chen S, Luo Q, Jiang Z, Wang X, Li Y, Qiu J, Yu K, Yang Y, Zhuang J (2022) Kruppel-like factor 2 acts as a tumor suppressor in human retinoblastoma. Exp Eye Res 216:108955. https://doi.org/10.1016/j.exer.2022.108955
Li J, Jiang JL, Chen YM, Lu WQ (2023) KLF2 inhibits colorectal cancer progression and metastasis by inducing ferroptosis via the PI3K/AKT signaling pathway. J Pathol Clin Res 9(5):423–435. https://doi.org/10.1002/cjp2.325
Li XM, Hu SJ, Liu JF, Ma MJ, Du LM, Bai FH (2023) Krüppel-like factor 2 is a gastric cancer suppressor and prognostic biomarker. Evid-Based Complement Altern Med: eCAM. https://doi.org/10.1155/2023/2360149
Liu CY, Chang TH, Hsieh CH, Chang YH, Pang JS, Chuang CK (2022) Kruppel-like factor 2 inhibits proliferation in renal angiomyolipoma via IL-6/JAK/STAT3 signaling pathway. Anticancer Res 42(10):4753–4762. https://doi.org/10.2187/anticanres.15980
Gao J, Hu J, Yu F, Wang C, Sheng D, Liu W, Hu A, Yu K, Xiao X, Kuang Y, Zacksenhaus E, Gajendran B, Ben-David Y (2023) Lovastatin inhibits erythroleukemia progression through KLF2-mediated suppression of MAPK/ERK signaling. BMC Cancer 23(1):306. https://doi.org/10.1186/s12885-023-10742-4
Zhu KY, Tian Y, Li YX, Meng QX, Ge J, Cao XC, Zhang T, Yu Y (2022) The functions and prognostic value of Krüppel-like factors in breast cancer. Cancer Cell Int 22(1):23. https://doi.org/10.1186/s12935-022-02449-6
Wang C, Sample KM, Gajendran B, Kapranov P, Liu W, Hu A, Zacksenhaus E, Li Y, Hao X, Ben-David Y (2021) FLI1 induces megakaryopoiesis gene expression through WAS/WIP-dependent and independent mechanisms; implications for wiskott-aldrich syndrome. Front Immunol 12:607836. https://doi.org/10.3389/fimmu.2021.607836
Crown J, O’Leary M, Ooi WS (2004) Docetaxel and paclitaxel in the treatment of breast cancer: a review of clinical experience. Oncologist 9(2):24–32. https://doi.org/10.1634/theoncologist.9-suppl_2-24
Kunder R, Velyunskiy M, Dunne SF, Cho BK, Kanojia D, Begg L, Orriols AM, Fleming-Trujillo E, Vadlamani P, Vialichka A, Bolin R, Perrino JN, Roth D, Clutter MR, Zielinski-Mozny NA, Goo YA, Cristofanilli M, Mendillo ML, Vassilopoulos A, Horiuchi D (2022) Synergistic PIM kinase and proteasome inhibition as a therapeutic strategy for MYC-overexpressing triple-negative breast cancer. Cell Chem Biol 29(3):358–372. https://doi.org/10.1016/j.chembiol.2021.08.011
Landor SKJ, Santio NM, Eccleshall WB, Paramonov VM, Gagliani EK, Hall D, Jin SB, Dahlström KM, Salminen TA, Rivero-Müller A, Lendahl U, Kovall RA, Koskinen PJ, Sahlgren C (2021) PIM-induced phosphorylation of Notch3 promotes breast cancer tumorigenicity in a CSL-independent fashion. J Biol Chem 296:100593. https://doi.org/10.1016/j.jbc.2021.100593
Zhao W, Qiu R, Li P, Yang J (2017) PIM1: a promising target in patients with triple-negative breast cancer. Med Oncol 34(8):142. https://doi.org/10.1007/s12032-017-0998-y
Sawaguchi Y, Yamazaki R, Nishiyama Y, Mae M, Abe A, Nishiyama H, Nishisaka F, Ibuki T, Sasai T, Matsuzaki T (2021) Novel pan-pim kinase inhibitors with imidazopyridazine and thiazolidinedione structure exert potent antitumor activities. Front Pharmacol 12:672536. https://doi.org/10.3389/fphar.2021.672536
Rajan AM, Kumar S (2016) New investigational drugs with single-agent activity in multiple myeloma. Blood Cancer J 6(7):e451. https://doi.org/10.1038/bcj.2016.53
Brasó-Maristany F, Filosto S, Catchpole S, Marlow R, Quist J, Francesch-Domenech E, Plumb DA, Zakka L, Gazinska P, Liccardi G, Meier P, Gris-Oliver A, Cheang MC, Perdrix-Rosell A, Shafat M, Noël E, Patel N, McEachern K, Scaltriti M, Castel P, Noor F, Buus R, Mathew S, Watkins J, Serra V, Marra P, Grigoriadis A, Tutt AN (2016) PIM1 kinase regulates cell death, tumor growth and chemotherapy response in triple-negative breast cancer. Nat Med 22(11):1303–1313. https://doi.org/10.1038/nm.4198
Mung KL, Eccleshall WB, Santio NM, Rivero-Müller A, Koskinen PJ (2021) PIM kinases inhibit AMPK activation and promote tumorigenicity by phosphorylating LKB1. Cell Commun Signal: CCS 19(1):68. https://doi.org/10.1186/s12964-021-00749-4
Wang B, Liu M, Song Y, Li C, Zhang S, Ma L (2019) KLF2 inhibits the migration and invasion of prostate cancer cells by downregulating MMP2. Am J Men’s Health 13(1):1557988318816907. https://doi.org/10.1177/1557988318816907
Shamloo B, Usluer S (2019) p21 in Cancer research. Cancers. https://doi.org/10.3390/cancers11081178
Yu ZH, Chen ZH, Zhou GL, Zhou XJ, Ma HY, Yu Y, Wang X, Cao XC (2022) miR-92a-3p promotes breast cancer proliferation by regulating the KLF2/BIRC5 axis. Thorac Cancer 13(21):2992–3000. https://doi.org/10.1111/1759-7714.14648
Acknowledgments
Not applicable.
Funding
This research was funded by research grants from the National Natural Science Foundation of China (U1812403 and 82260040) to Yaacov Ben-David, the National Natural Science Foundation of China (82060239 and GPPHNSFC-2020-9), Guizhou Provincial Natural Science Foundation of China (Qiankehejichu-ZK [2021] Zhongdian006) and Guizhou Provincial High-level creative talents cultivation plan: Thousand plan (GZSYQCC [2016]001) and Guangzhou Provincial Medical Science Basic Research (B2023047) to Xiangdi Yu, the Science and Technology Department of Guizhou Province (QKHJC-ZK[2024] Yiban 641) and the Guizhou Administration of Traditional Chinese Medicine (QZYY-2024-023) to Anling Hu, the Open Grant from State Key Laboratory for Functions and Applications of Medicinal Plants of Guizhou Medical University (QJHKYZ [2022]391) and the Science and Technology Department of Guizhou Province Grants (QKHJC-ZK [2022] YB297) to Xiao Xiao, and the Science and Technology Department of Guizhou Province Grants (QKHJC-ZK[2023]YB240) to Chunlin Wang.
Author information
Authors and Affiliations
Contributions
X.R., A.H., C.W., Y.K., and X.X. contributed to the conception, design of the study, as well as methodology, data acquisition, and interpretation. W.L. and X.X. were involved in the statistical analysis. X.R. and A.H. drafted the manuscript. Y.BD., X.Y., and E.Z. reviewed the manuscript critically. Y.BD. and X.Y. supervised, conceived, funding acquisition, and designed the study. All authors contributed to the interpretation of the findings, reviewed, edited, and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interest
The authors have declared that no competing interest exists.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Research involving human and animal participant
Animal care and procedures followed by the criteria for the use of laboratory animals. The animal protocol was reviewed and approved by the Guizhou Medical University Animal Care Committee under the guidelines of the China Council of Animal Care (Approval ID #1900373).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10549_2024_7372_MOESM1_ESM.tif
Supplementary file1 (TIF 1471 KB)—Fig.1 UM171 induces apoptosis and cell cycle arrest of MDA-MB-231 breast cancer cells in culture (a) The apoptosis index of MDA-MB-231 cells after treatment for 24 h with the indicated concentration of UM171. (b) The cell cycle analysis of MDA-MB-231 cells after treatment with the indicated doses of UM171 for 24h.
10549_2024_7372_MOESM2_ESM.tif
Supplementary file2 (TIF 1018 KB)—Fig.2 UM171 induces apoptosis and cell cycle arrest of 4T1 breast cancer cells in culture (a) The apoptosis index of 4T1 cells after treatment for 24 h with the indicated concentration of UM171. (b) The cell cycle analysis of 4T1 cells after treatment with the indicated doses of UM171 for 24 h.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ran, X., Hu, A., Kuang, Y. et al. UM171 suppresses breast cancer progression by inducing KLF2. Breast Cancer Res Treat (2024). https://doi.org/10.1007/s10549-024-07372-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10549-024-07372-0