Abstract
Purpose
Breast cancer (BC) in women under 45 is rare yet often aggressive. We aim to analyze loco-regional recurrences (LR), distant recurrences (DR), second breast cancers, and mortality in young BC patients.
Methods
We enrolled 776 women with non-metastatic BC ≤45 years diagnosed from 1970 to 2012. Variables included age, family history, tumor stage/grade, and treatment. We used multivariate Cox regression and competing risk models.
Results
Among the participants, 37.0% were diagnosed before the age of 40. Most had stage I or II, grade II, ER- and PR-positive, HER2-negative tumors. Over a median follow-up of 8.7 years, 10.1% experienced LR, 13.7% developed DR, and 10.8% died, primarily due to BC. The majority of recurrences occurred within the first five years. Older age (>40) significantly reduced the risk of LR and DR. Advanced disease stage, certain surgical strategies, and positive margins increased DR risk. In the cohort diagnosed between 2001 and 2012, recent diagnosis, triple-negative cancer, and hormonal therapy were associated with reduced LR risk. Breast-conserving surgery appeared to offer protective effects against DR.
Conclusion
This study highlights that BC in young women carries a significant risk of early recurrence, with age, tumor characteristics, and treatment modalities influencing outcomes. The findings emphasize the need for tailored treatment strategies for young BC patients, focusing on surgical precision and aggressive adjuvant therapy for high-risk cases. This research contributes valuable insights into managing BC in younger patients, aiding in improving long-term outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
A breast cancer (BC) diagnosis in young women is relatively rare. In 2020, the age-standardized incidence rate in Europe for women aged 45 and older was estimated at 228.6 per 100,000, compared to 20.1 per 100,000 for those 45 years or younger [1]. Breast cancer is the leading cause of cancer death among women under 45 [1].
Young women with BC typically present with larger, more aggressive, and advanced tumors, leading to shorter survival [2]. They have a higher risk of developing recurrences [3,4,5]. Particularly, loco-regional recurrences (LR) in this group have been linked to increased risks of distant metastasis and mortality [6]. BC subtypes also influence recurrence patterns. HER2-positive and triple-negative tumors, more common in younger women, are prone to local and distant recurrences (DR) [2, 7].
The risk of loco-regional and distant recurrences for young women with breast cancer has not been extensively investigated. Young patients with breast cancer usually represent a very small subset of eligible patients in randomized clinical trials and observational study populations, the latter often reporting only on hospital-selected patients [8]. Few population-based studies have specifically addressed this demographic [7, 9, 10].
A study by Aalders et al. noted a decline in 5-year local and regional recurrence rates in patients under 35 from 2003 to 2008, attributing this improvement to advancements in treatment and management [11]. The role of systemic therapy and targeted drugs in reducing recurrence risk is acknowledged, but longer follow-up is needed for accurate risk assessment in very young women.
Furthermore, when estimating the absolute risk of recurrence and death for clinical purposes or risk stratification, it is important to consider that these events are not independent from each other and that the estimation without considering competing risks could be biased. In general, it tends to overestimate the risk and the bias increases with length of follow-up and for less common end-points as local recurrences (LR) [12].
We constituted a retrospective population-based cohort of breast cancer women aged ≤45 years in Geneva, Switzerland, with the purpose of evaluating tumor characteristics, patterns of care, prognostic factors, and outcomes of breast cancer in this population [13]. In the present article, we use competing risk analysis to describe the long-term patterns of loco-regional recurrences, distant recurrences, second breast cancer, and death in this cohort of young women. This approach allows us to avoid biased risk estimates. In addition, we investigated factors associated with the development of recurrences.
Methods
The cohort has been enrolled using the population-based Geneva Cancer Registry (GCR), which records since 1970 all incident cancers occurring in the population of the county (approximately 500,000 inhabitants in 2023). The detail study protocol has been previously described [13]. In brief, all women resident in the canton of Geneva and diagnosed with non-metastatic primary invasive breast cancer (stages I–III) at the age of 45 years or less between 1970 and 2012 were enrolled in the cohort. Women with previous or synchronous invasive cancer were not included.
Data routinely registered from the GCR include patient, tumor, and treatment characteristics. Pathological and medical files were reviewed to collect additional data, such as family history of breast cancer and recurrences. As estrogen and progesterone receptor (ER and PR) status recording began in 1995, the study was limited to patients diagnosed from 1995 to 2012 who underwent surgical treatment.
Individual characteristics of interest were age at diagnosis, year of diagnosis, and family history of breast and/or ovarian cancer. Familial risk was categorized as high (at least one first-degree relative with breast/ovarian cancer diagnosed before the age of 50 years, or at least two first-degree relatives with breast/ovarian cancer at any age, or at least three cases of breast/ovarian cancer among first- or second-degree relatives), low (no affected first- or second-degree relatives with breast/ovarian cancer), or moderate (all other known family histories) according to a previous study by our group [14]. Tumor variables considered were tumor stage and tumor differentiation coded according to the TNM using the pathological classification and when missing the clinical classification [15]. ER and PR status were categorized as positive (when at least 1% of the receptors were positive), negative, or unknown. The information about the Ki67 and HER2 status were collected only since 2000 and 2001, respectively. Therefore, we were able to classify tumor biology as expressed by tumor biomarker subtypes only for the subset of women diagnosed since 2001. Based on ER and PR status, Ki67, expression of HER2 and grade, four breast cancer surrogate subtypes were defined [16, 17]: Luminal A-like: (Ki67 <14%, or if unknown grade I or II, HER2−, ER+, and/or PR+); Luminal B-like (any Ki67 or any grade, HER2+, ER+, and/or PR+; Ki67 ≥14%, or if unknown grade III, HER2−, ER+, and/or PR+); HER2+/HR− (HER2+, ER−, and PR−); and Triple negative (HER2−, ER−, and PR−). The surgical treatments under consideration included breast-conserving surgery with radiotherapy, mastectomy without radiotherapy, mastectomy with radiotherapy, and other combination of surgical procedures. Additionally, we assessed the administration of chemotherapy (Yes/No) and hormonal treatment (Yes/No).
Women were followed for loco-regional and distant recurrences, second breast cancer occurrence or death up to 31/12/2015. The information about LR and DR were collected from the clinical files of the patients. LR was defined as any breast cancer in surgical scar, in skin and subcutaneous tissue on the ipsilateral breast and thoracic wall if the morphology was similar as the primary tumor, in ipsilateral axillary, infraclavicular, supraclavicular, internal mammary/parasternal, or intramammary lymph node. DR was defined as breast cancer in any organ other than the breast, excluding the events listed under LR. Information about the second breast cancer was collected from the GCR. Second breast cancer was defined as any epithelial breast cancer, in situ or invasive, in the contralateral breast, or in the ipsilateral breast if of different morphologies from the first one [18]. Vital status was ascertained through passive (hospital and mortality certificates) and active follow-up using the files of the Cantonal Population Office in charge of registration of the resident population of the Canton.
Cumulative incidence was estimated for LR and DR in a competing risks framework which considered second breast cancer and deaths from all causes as competing events. Analyses were further stratified by age, stage, grade, and ER status.
To investigate factors associated with the occurrence of LR and DR in the presence of competing risk events, including second cancer occurrences and mortality, we conducted multivariate Cox regression analyses on a complete case basis. This method involved only participants with complete data on all variables of interest. Our analyses considered a predefined set of covariates selected a priori based on their established prognostic impact. These analyses were applied to two distinct cohorts: the whole cohort, with all the cases diagnosed between 1995 and 2012, and the cohort of women diagnosed 2001 and 2012 for whom it was available the surrogate molecular subtype. The covariates, chosen based on prior knowledge of their relevance, were age at diagnosis, year of incidence (continuous), family history, disease stage, grade, ER status (for the 1995–2012 cohort) or surrogate molecular type (for the 2001–2012 cohort), surgical treatment strategy, surgical margins, and the administration of chemotherapy and hormonal treatment.
Given that we are operating within a competing risk setting, it is important to note that these LR and DR models are inherently interconnected, as understanding the risk factors for one event may provide insights into the occurrence of the other event.
All the results were considered statistically significant at a p value < 0.05. All the analyses were performed using STATA software (version 17, [19]).
Results
A total of 776 young women with breast cancer were included in the study. Patient, tumor, and treatment characteristics of the women in this cohort are shown according to the type of first event experienced (Table 1).
Breast cancer was diagnosed before the age of 40 years in 287 women (37.0%) and between 40 and 45 years in 489 women (63.0%). Most women had no family history of breast and/or ovarian cancer (63.5%). Most tumors were diagnosed at stage I (n=301, 39.8%) or II (n=362, 47.8%) and of grade II (n=365, 48.1%), were ER-positive and PR-positive (n=586, 77.3% and n=528, 68.0%, respectively), HER2-negative (n=386, 76.4%), and of luminal A-like or luminal B-like molecular subtype (n=200, 39.8% and n=201, 42.1%, respectively). Most women were treated with chemotherapy (n=586, 76.3%) and hormonotherapy (n=516, 68.2%) and had breast-conserving surgery (BCS) as surgical strategy (n=489, 64.2%).
At the end of the follow-up (median: 8.7 years), most women (n=587, 75.64%) did not experience any of the study events; 78 women (10.1%) had at least one LR, 106 (13.7%) developed a DR, 36 (4.6%) were diagnosed with a second primary breast cancer, and 84 women (10.8%) died during the study period, 68 of whom from BC. The median time to the first event was 7.3 years.
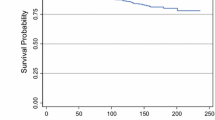
The competing risk analysis considering the occurrence of the first event in presence of competing risk events showed that recurrences occurred throughout the follow-up period. After 5, 10, and 15 years of follow-up, the cumulative incidence of LR was 5.5% [95% CI: 3.9–7.2], 9.4% [95% CI: 7.1–11.8], and 13.3% [95% CI: 10.0–17.1] that of DR was 6.5% [95% CI: 4.8–8.4], 11.1% [95% CI: 8.4–13.5], and 14.1% [95% CI: 10.4–17.1]. (Fig. 1). Most of the recurrences occurred during the first 5 years of follow-up (57% for LR and 62% for DR, respectively).
The cumulative incidence rates for LR were very similar between women aged less than 40 and 40–45 during the first two years after diagnosis and then younger women showed higher rates although never statistically different from women 40 to 45 years old (Suppl. Fig. 1a). For DR, already one year after diagnosis, younger women showed higher rates, and at 5 years, their cumulative incidence was 9. 3% [95% CI: 6.1–13.2] vs. 5.0% [95% CI: 3.3–7.3] for women 40–45 years old (Suppl. Fig. 1b).
No statistical differences were found for LR according to stage at diagnosis during the first 5 years of follow-up, although a trend toward higher incidence rates with higher stage was observed (Suppl. Fig. 2a). For DR, stage III tumors had a cumulative incidence rate always higher than tumors with lower stages at diagnosis, at 5 years being 15.1% (95% CI: 8.5–23.5) compared to 2.9% (95% CI: 1.4–5.5) for stage I tumors (Suppl. Fig. 2b).
The cumulative incidence of LR according to grade at diagnosis showed clear differences, with grade I tumors presenting very low rates of recurrence up to 5 years (0.7%, 95% CI: 1–3.7), while grade III tumors presented a rate of almost 8% (95% CI: 4.9–11.8) and grade II tumors rates were in between the two (Suppl. Fig. 3a). On the contrary, for DR, although a gradient with increasing grade was seen, the differences were not statistically significant (Suppl. Fig. 3b).
The cumulative incidence according to ER status showed a higher loco-regional rate in the first 5 years of follow-up among women ER-negative compared to women with ER-positive tumors, although not statistically significant. (Suppl. Fig. 4a). The cumulative incidence of DR increased steeply during the first years after diagnosis for ER-negative tumors (11.7% at 5 years, 95% CI: 7.3–17.2) compared with ER-positive tumors (4.9%, 95% CI: 3.3–6.9). (Suppl. Fig. 4b).
In the multivariate Cox analysis of the whole cohort of women diagnosed between 1995 and 2012, the only factor that showed a significant association with LR development as first event was age. Notably, women aged 40 and older presented a reduced hazard ratio (HR) of 0.51 (95% CI: 0.30–0.87) compared to women who were <40 years old at diagnosis. Similarly, older women showed a significant decreased risk of developing DR as first event (HR: 0.47, 95% CI: 0.28–0.78). Other factors associated with DR were a more advanced stage (HR of stage III vs stage I: 3.06, 95% CI: 1.25–7.52), surgical strategy (HR of mastectomy plus RT vs BCS plus RT: 2.21, 95% CI: 1.18–4.14), and positive margins after surgery (HR: 2.88, 95%CI: 1.28–6.49) (Table 2).
When analyzing the cohort diagnosed during the period 2001–2012, three factors resulted significantly associated with a reduced risk of developing LR as a first event: period of diagnosis (HR: 0.83, 95% CI: 0.72–0.96); molecular subtype triple negative (HR: 0.16 as compared to Luminal A-like, 95% CI: 0.03–0.92); and treatment with hormonal therapy (HR: 0.25, 95% CI: 0.08–0.76). For DR as a first event, women in this cohort showed a lower risk if older than 40 years (HR: 0.41, 95% CI: 0.2-0-0.83) and higher risk if stage III at diagnosis (HR of stage III vs stage I: 4.99, 95% CI: 1.51–16.5); luminal B molecular type (HR: 3.17, 95% CI:1.18–8.55); had mastectomy plus RT as surgical strategy (HR: 3.44, 95% CI: 1.44–8.22); and positive margins after surgery (HR: 5.39, 95%CI: 1.70–17.06) (Table 2). Triple-negative tumors presented a HR of 6.21 compared to the reference Luminal A-like tumors, but the result did not reach statistical significance (p=0.054).
Conclusion
In this cohort of women diagnosed with breast cancer at the age of 45 years or less followed a median of seven years, more than one-fourth experienced one of the study outcomes, including LR and DR, second breast cancer, or death as the first event. DR occurred only slightly more often than LR all along the follow-up period, and the risk remained important still at 10 and 15 years after the diagnosis. The timing and rate of LR and DR varied with age, tumor characteristics, and type of surgery. A lower risk of developing LR as first event was associated with older age on the overall cohort, and among women diagnosed more recently, with period of diagnosis, triple-negative molecular subtype, and use of hormonal therapy. Older age was also associated with a lower risk of developing DR, while more advanced stage, type of surgery strategy, positive margins after surgery, and molecular subtype of the tumor, for the subset of women for whom this could be defined, were all associated with a higher risk.
In the POSH longitudinal cohort study, Maishman et al. found that the frequency of LR among young breast cancer patients <40 years old at diagnosis was much lower than that of DR, at least within the first 10 years [20]. In our cohort, although DR were more frequent, the difference was very small, likely because local and regional recurrences were considered together.
Although the recurrences occurred all along the follow-up period, the majority of them was diagnosed during the first 5 years of follow-up. As showed for older breast cancer patients [21], the occurrence among young women seems to present two peaks, one during the first two years of follow-up and a second one at 5–7 years [22]. The higher risk of developing both LR and DR within the first two years of diagnosis was observed in our cohort among women younger than 40 years, women with stage III disease or with ER-negative cancers.
Younger age at diagnosis showed up as an independent factor associated with the development of LR as first event when using the whole cohort. This result is in line with most studies [3, 23]. Of note are the results of the analysis carried out on the sub-cohort of women diagnosed between 2001 and 2012 that showed a lower risk among women carrying triple-negative cancers to develop LR. This finding is consistent with those of a previous study of triple-negative breast cancer patients showing that these cancers more frequently develop a systemic recurrence, while a loco-regional recurrence occur more rarely [24, 25]. The concurrent much higher risk of DR, although not statistically significant, among triple-negative breast cancers observed in the multivariate model for the cohort of women diagnosed in 2001–2012 in the present study, strongly supports this argument.
In addition, our results showed a decreased risk of LR among women treated with hormonal therapy. While tamoxifen has been the standard of therapy for ER/PR-positive breast cancers regardless of age for many years, recently the use of aromatase inhibitors and the addition of Ovarian Function Suppression (OFS) have been included in the treatment of young women [26]. We could collect the type of treatment received for 491 out of the 776 women who had a hormonal treatment. Among them, 96% in the period 1995–2000 received tamoxifen, while in the period 2001–2012 80% received tamoxifen, 10% received an aromatase, alone or after tamoxifen, and 9% had also surgical or medical OFS.
As in other studies, we found that factors influencing the risk of DR were age, stage at diagnosis, and molecular subtype for the subset of women for whom this could be defined [7, 27].
Positive surgical margins after surgery were an independent factor increasing the risk of DR, in total agreement with the results of two recent systematic reviews and meta-analyses that showed that involved or close pathological margins are associated with an increased risk of local and distant recurrences both after breast-conserving surgery [28] and mastectomy [29].
Type of surgery strategy was also an important determinant of recurrence risk in several studies. Recently, Pederson et al. reported a lower risk of late breast cancer recurrences among patients who received BCS compared to those who received a mastectomy [27]. Similarly, Elder et al. in their study [30] and Ho-Huynh et al. in their systematic review of breast cancer outcomes in Australia [31] showed that women who underwent mastectomy were at increased risk of recurrence compared to women who underwent BCS. In our study, we also found that BCS had a similar or even protective effect toward the risk of DR in a multivariate model, confirming that young age is not a contraindication for BCS [32]. Post-mastectomy radiation therapy is indeed recommended for patients at high risk of recurrence, including those with involved axillary lymph nodes, positive resection margins, and T3–T4 tumors independent of nodal status [33]. The higher risk of DR confined to those patients who received both mastectomy and radiotherapy may indicate that these patients were indeed at higher risk and that our models do not fully adjust for all the factors that increase this risk.
This study has several strengths. It is a population-based cohort in Geneva of all women ≤45 years old diagnosed with breast cancer in a recent period with a follow-up to 20 years for death and recurrences. The study variables, including recurrence diagnosis, have been collected with high accuracy. Thanks to a strong and high-quality network acting in a restricted geographical area, we are confident that all available information is captured. Therefore, the assumption “no information, no recurrences” is trustworthy. This assumption was tested and validated for a 30 cases random sample for which active search was performed using all available sources, including direct contact with patients’ physicians. Furthermore, we used competing risk analysis that allowed to correctly estimate the absolute risk of recurrence in the population of young women, unbiased by other competing risk events.
The main limitation of the study is related to its observational nature. We had a lot of information available about each woman, and in the analysis of the determinants of recurrence, we included many of the variables considered important for the outcome of interest; however, we cannot assure to have eliminated all the possible bias and confounding. The small numbers of women in some subgroups did not allow for more detailed analysis. Our focus was on the first event and did not consider the risk of developing more than one event. Another limitation is the fact that not all the patients were classified according to surrogate molecular subtypings due to the lack of information on HER2 and Ki67 status before 2001. Finally, these findings are not directly applicable to current patients since standardized treatments offered now maybe different compared to those offered in the period under study.
Due to the increasing number of women being diagnosed with BC, also of young age, and the increasing number of long-term survivors, the estimation of the risk of recurrence and the identification of factors related to development of recurrence are of extreme importance. The prognosis of patients with recurrences, particularly distant recurrences, is less favorable and proper adjustments in the management of young patients are necessary to improve their outcomes.
Special attention is required to the type of surgery offered and the achievement of negative margins, as well as more aggressive adjuvant therapy in case of more advanced or poorly differentiated tumors, such as receptor-negative and/or HER2-positive tumors.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. In compliance with data protection regulations, data are stored at the Geneva Cancer Registry, Geneva, Switzerland, but are available from the corresponding author on reasonable request.
Abbreviations
- BC:
-
Breast cancer
- DR:
-
Distant recurrence
- LR:
-
Loco-regional recurrence
- HR:
-
Hazard ratio
- GCR:
-
Geneva cancer registry
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- BCS:
-
Breast-conserving surgery
References
Global Cancer Observatory. https://gco.iarc.fr/. Accessed 15 June 2020
Narod SA (2012) Breast cancer in young women. Nat Rev Clin Oncol 9:460–470. https://doi.org/10.1038/nrclinonc.2012.102
Voogd AC, Nielsen M, Peterse JL et al (2001) Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: pooled results of two large European randomized trials. J Clin Oncol 19:1688–1697. https://doi.org/10.1200/JCO.2001.19.6.1688
de Bock GH, van der Hage JA, Putter H et al (2006) Isolated loco-regional recurrence of breast cancer is more common in young patients and following breast conserving therapy: long-term results of European Organisation for Research and Treatment of Cancer studies. Eur J Cancer 42:351–356. https://doi.org/10.1016/j.ejca.2005.10.006
de Bock GH, Putter H, Bonnema J et al (2009) The impact of loco-regional recurrences on metastatic progression in early-stage breast cancer: a multistate model. Breast Cancer Res Treat 117:401–408. https://doi.org/10.1007/s10549-008-0300-2
Laurberg T, Lyngholm CD, Christiansen P et al (2016) Long-term age-dependent failure pattern after breast-conserving therapy or mastectomy among Danish lymph-node-negative breast cancer patients. Radiother Oncol 120:98–106. https://doi.org/10.1016/j.radonc.2016.05.006
Laurberg T, Alsner J, Tramm T et al (2017) Impact of age, intrinsic subtype and local treatment on long-term local-regional recurrence and breast cancer mortality among low-risk breast cancer patients. Acta Oncol 56:59–67. https://doi.org/10.1080/0284186X.2016.1246803
He X-M, Zou D-H (2017) The association of young age with local recurrence in women with early-stage breast cancer after breast-conserving therapy: a meta-analysis. Sci Rep 7:11058. https://doi.org/10.1038/s41598-017-10729-9
Cao JQ, Truong PT, Olivotto IA et al (2014) Should women younger than 40 years of age with invasive breast cancer have a mastectomy? 15-year outcomes in a population-based cohort. Int J Radiat Oncol Biol Phys 90:509–517. https://doi.org/10.1016/j.ijrobp.2014.06.041
van der Sangen MJC, van de Wiel FMM, Poortmans PMP et al (2011) Are breast conservation and mastectomy equally effective in the treatment of young women with early breast cancer? Long-term results of a population-based cohort of 1,451 patients aged ≤ 40 years. Breast Cancer Res Treat 127:207–215. https://doi.org/10.1007/s10549-010-1110-x
Aalders KC, Postma EL, Strobbe LJ et al (2016) Contemporary Locoregional Recurrence Rates in Young Patients With Early-Stage Breast Cancer. J Clin Oncol 34:2107–2114. https://doi.org/10.1200/JCO.2015.64.3536
Saleh RR, Nadler MB, Desnoyers A et al (2020) Influence of competing risks on estimates of recurrence risk and breast cancer-specific mortality in analyses of the early breast cancer Trialists Collaborative Group. Sci Rep 10:4091. https://doi.org/10.1038/s41598-020-61093-0
Schaffar R, Bouchardy C, Chappuis PO et al (2019) A population-based cohort of young women diagnosed with breast cancer in Geneva. Switzerland. PLoS ONE 14:e0222136. https://doi.org/10.1371/journal.pone.0222136
Verkooijen HM, Chappuis PO, Rapiti E et al (2006) Impact of familial risk factors on management and survival of early-onset breast cancer: a population-based study. Br J Cancer 94:231–238. https://doi.org/10.1038/sj.bjc.6602914
TNM Classification of Malignant Tumours, 8th Edition | Wiley. In: Wiley.com. https://www.wiley.com/en-gb/TNM+Classification+of+Malignant+Tumours%2C+8th+Edition-p-9781119263579. Accessed 17 Feb 2023
Goldhirsch A, Wood WC, Coates AS et al (2011) Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 22:1736–1747. https://doi.org/10.1093/annonc/mdr304
Arvold ND, Taghian AG, Niemierko A et al (2011) Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol 29:3885–3891. https://doi.org/10.1200/JCO.2011.36.1105
Moossdorff M, van Roozendaal LM, Strobbe LJA et al (2014) Maastricht Delphi consensus on event definitions for classification of recurrence in breast cancer research. J Natl Cancer Inst 106. https://doi.org/10.1093/jnci/dju288
(2021) Stata Statistical Software
Maishman T, Cutress RI, Hernandez A et al (2017) Local recurrence and breast oncological surgery in young women with breast cancer: the POSH observational cohort study. Ann Surg 266:165–172. https://doi.org/10.1097/SLA.0000000000001930
Jatoi I, Tsimelzon A, Weiss H et al (2005) Hazard rates of recurrence following diagnosis of primary breast cancer. Breast Cancer Res Treat 89:173–178. https://doi.org/10.1007/s10549-004-1722-0
Geurts YM, Witteveen A, Bretveld R et al (2017) Patterns and predictors of first and subsequent recurrence in women with early breast cancer. Breast Cancer Res Treat 165:709–720. https://doi.org/10.1007/s10549-017-4340-3
Veronesi U, Cascinelli N, Mariani L et al (2002) Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 347:1227–1232. https://doi.org/10.1056/NEJMoa020989
Van Roozendaal LM, Smit LHM, Duijsens GHNM et al (2016) Risk of regional recurrence in triple-negative breast cancer patients: a Dutch cohort study. Breast Cancer Res Treat 156:465–472. https://doi.org/10.1007/s10549-016-3757-4
James M, Dixit A, Robinson B et al (2019) Outcomes for patients with non-metastatic triple-negative breast cancer in New Zealand. Clin Oncol (R Coll Radiol) 31:17–24. https://doi.org/10.1016/j.clon.2018.09.006
Tesch ME, Partridge AH (2022) Treatment of breast cancer in young adults. Am Soc Clin Oncol Educ Book 42:1–12. https://doi.org/10.1200/EDBK_360970
Pedersen RN, Esen BÖ, Mellemkjær L et al (2022) The incidence of breast cancer recurrence 10–32 years after primary diagnosis. J Natl Cancer Inst 114:391–399. https://doi.org/10.1093/jnci/djab202
Bundred JR, Michael S, Stuart B et al (2022) Margin status and survival outcomes after breast cancer conservation surgery: prospectively registered systematic review and meta-analysis. BMJ 378:e070346. https://doi.org/10.1136/bmj-2022-070346
Bundred J, Michael S, Bowers S et al (2020) Do surgical margins matter after mastectomy? A systematic review. Eur J Surg Oncol 46:2185–2194. https://doi.org/10.1016/j.ejso.2020.08.015
Elder EE, Kennedy CW, Gluch L et al (2006) Patterns of breast cancer relapse. Eur J Surg Oncol 32:922–927. https://doi.org/10.1016/j.ejso.2006.06.001
Ho-Huynh A, Tran A, Bray G et al (2019) Factors influencing breast cancer outcomes in Australia: a systematic review. Eur J Cancer Care (Engl) 28:e13038. https://doi.org/10.1111/ecc.13038
Sun Z-H, Chen C, Kuang X-W et al (2021) Breast surgery for young women with early-stage breast cancer: mastectomy or breast-conserving therapy? Medicine (Baltimore) 100:e25880. https://doi.org/10.1097/MD.0000000000025880
Cardoso F, Kyriakides S, Ohno S et al (2019) Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol 30:1194–1220. https://doi.org/10.1093/annonc/mdz173
Acknowledgements
This study was funded by the Swiss Cancer League (KFS-3713-08-2015). We thank all the registrars that contributed to the data collection.
Funding
Open access funding provided by University of Geneva. The study was funded by the Swiss Cancer League (KFS-3713-08-2015).
Author information
Authors and Affiliations
Contributions
RS, SB, and ER developed the concept and initiated the study. RS conducted the statistical analysis. RS and ER drafted the manuscript. SB and POC helped to critically revise the draft for important intellectual content, read, and then approved the final version to be published.
All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Ethics approval and consent to participate
The Geneva Cancer Registry has a general authorization to collect nominative data and analyze the anonymized data. Ethics approval is therefore not needed. All methods were performed in accordance with the relevant guidelines and regulations (Declaration of Helsinki).
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schaffar, R., Benhamou, S., Chappuis, P.O. et al. Risk of first recurrence after treatment in a population-based cohort of young women with breast cancer. Breast Cancer Res Treat (2024). https://doi.org/10.1007/s10549-024-07338-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10549-024-07338-2