Abstract
Purpose
UK NICE guidelines recommend axillary node clearance (ANC) should be performed in all patients with biopsy-proven node-positive breast cancer having primary surgery. There is, however, increasing evidence such extensive surgery may not always be necessary. Targeted axillary dissection (TAD) may be an effective alternative in patients with low-volume nodal disease who are clinically node negative (cN0) but have abnormal nodes detected radiologically. This survey aimed to explore current management of this group to inform feasibility of a future trial.
Methods
An online survey was developed to explore current UK management of patients with low-volume axillary disease and attitudes to a future trial. The survey was distributed via breast surgery professional associations and social media from September to November 2022. One survey was completed per unit and simple descriptive statistics used to summarise the results.
Results
51 UK breast units completed the survey of whom 78.5% (n = 40) reported performing ANC for all patients with biopsy-proven axillary nodal disease having primary surgery. Only 15.7% of units currently performed TAD either routinely (n = 6, 11.8%) or selectively (n = 2, 3.9%). There was significant uncertainty (83.7%, n = 36/43) about the optimal surgical management of these patients. Two-thirds (n = 27/42) of units felt an RCT comparing TAD and ANC would be feasible.
Conclusions
ANC remains standard of care for patients with low-volume node-positive breast cancer having primary surgery in the UK, but considerable uncertainty exists regarding optimal management of this group. This survey suggests an RCT comparing the outcomes of TAD and ANC may be feasible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Breast cancer affects almost 56,000 women every year in the United Kingdom (UK) [1], the majority of whom will have surgery as their first treatment. As part of their diagnostic assessment, all UK patients will have an ultrasound scan (USS) of their axilla with biopsy of any suspicious/indeterminate nodes to establish whether the cancer has spread and inform the type of axillary surgery that is performed. Current National Institute of Health and Care Excellence (NICE) guidance states that all women with biopsy-proven node-positive breast cancer should be offered an axillary node clearance (ANC), irrespective of the extent of disease [2].
ANC is a highly morbid procedure with one in three women experiencing significant, lifelong complications including lymphoedema and chronic pain that can dramatically impact their quality of life [3, 4]. Furthermore, there is no evidence that this extensive surgery improves breast cancer survival [5,6,7,8,9,10,11,12,13]. Internationally, the use of ANC is declining [14] based on the results of the landmark Z0011 study which showed that ANC did not improve survival in patients with clinically node-negative (cN0) disease found to have 1–2 involved nodes on sentinel node biopsy (SNB) [5,6,7,8,9] in women with T1 or T2 invasive primary breast cancer. In the UK, exploring alternatives to ANC in patients with node-positive breast cancer was identified as the top research priority in breast cancer surgery in a recent James Lind Alliance Priority Setting Partnership [15].
Targeted axillary dissection (TAD) is a new procedure that may provide an effective alternative to ANC in patients with low-volume nodal disease—i.e. those who are cN0 but have 1–2 involved nodes detected on USS. TAD combines a sentinel node biopsy with targeted removal of the involved node(s) that are localised prior to surgery. Use of TAD addresses concerns about high false-negative rates with SNB alone in patients with nodal involvement [16], facilitating removal of known disease, and providing accurate staging information to guide adjuvant treatment decision making whilst allowing patients to avoid the morbidity associated with an ANC. The technique has been shown to be feasible [17] and is now standard of care in node-positive patients who have a complete response to neoadjuvant chemotherapy [18]. There are also emerging data to support the use of TAD in patients having primary surgery [19].
However, there is a need to robustly evaluate TAD in the primary surgical setting before it is introduced into routine clinical practice. Ideally a randomised clinical trial (RCT) is needed but it is important that a future trial is well designed, reflects UK practice and addresses a question that is important to both clinicians and patients. This survey aims to explore the current management of patients with low-volume axillary nodal disease having primary surgery in the UK to inform the feasibility, design and conduct of a future RCT comparing surgical techniques.
Methods
An online national practice survey was developed in SurveyMonkey®. Questions explored the current management of patients with early breast cancer who have biopsy-proven low-volume axillary nodal disease defined as clinically node negative (cN0—on physical examination only) with no more than two suspicious nodes on axillary USS. Further questions focused on the feasibility and design of a future RCT comparing TAD and ANC in the primary surgery setting. (Appendix 1: National Practice Survey).
All breast surgery units in the UK were invited to complete the survey on behalf of their multidisciplinary teams (MDTs). The survey was distributed via social media and the UK breast surgery professional associations [Association of Breast Surgery (ABS) and The Mammary Fold, (UK trainee breast surgery group)] between September and November 2022. Regular reminders were sent via email and association newsletter to maximise participation. Simple descriptive statistics were used to summarise results.
Results
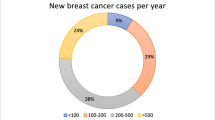
Surveys were completed by 51 UK breast units (of 130 UK units [20], 39%), most of whom treated between 201 and 600 new breast cancers per year (n = 36, 70%) (Table 1). For most centres, this included between 25 and 50 patients with low-volume axillary nodal disease annually (Table 1).
The majority of units (n = 40, 78.5%) reported that they would perform an ANC for patients with low-volume nodal disease having primary surgery. A minority of units (n = 8, 15.7%) reported performing TAD, either routinely (n = 6, 11.8%) or in certain circumstances (n = 2, 3.9%) and a few (n = 3, 5.8%) performed SNB in this context. Almost three quarters (38/51, 74.5%) of units were performing TAD in the neoadjuvant setting. Most units (n = 31/38, 81.6%) reported that a TAD comprised a dual tracer SNB combined with removal of pre-operatively localised node(s), although variations in the technique were highlighted (Table 2).
The majority of participating units (n = 36/43, 83.7%) reported uncertainty about the optimal surgical management of patients with low-volume nodal disease undergoing primary surgery and two thirds of MDTs (n = 27/42, 64.3%) felt that a future trial comparing TAD and ANC may be feasible. Most respondents felt that the trial should include both pre and post-menopausal patients with all molecular subtypes of breast cancer who had 1–2 involved nodes on USS (Table 3). Locoregional recurrence (LRR) was considered the most important primary outcome for a future trial by clinicians completing the survey.
Discussion
This survey suggests that ANC remains the standard of care for patients with low-volume nodal disease having primary surgery in the UK, in line with current NICE guidelines. Only a minority of units are currently offering TAD in this group, either routinely or in selected cases. There is, however, considerable uncertainty regarding optimal surgical management of these patients. A trial comparing TAD vs ANC is, therefore, necessary and likely to be feasible in the UK.
A number of previous studies including ACOSOG-Z0011 [5,6,7,8,9] and IBSCG 23-01 [10, 11], and several recent meta-analyses [12, 13], have shown no oncological benefit of ANC for cN0 patients with 1–2 involved nodes detected at sentinel node biopsy. These findings have been adopted into North American NCCN breast cancer guidelines [21] and have led to a decrease in the practice of ANC worldwide [22]. A number of confirmatory studies are ongoing including the UKANZ POSNOC [23] study, the results of which are awaited. Although patients in these studies fulfil the criteria for having ‘low volume nodal disease’; none of the studies aimed to address the optimal management of patients with biopsy-proven axillary nodal involvement at diagnosis. Node positivity in these studies was detected following surgical axillary staging (SNB) so these patients would be likely to have a lower burden of disease. A study specifically focusing on the management of patients with known node-positive disease at diagnosis comparing targeted removal of the abnormal nodes versus an ANC (the current standard of care), is needed to determine whether de-escalation of axillary surgery in patients with low-volume nodal disease is safe and effective.
TAD has been shown to be feasible [17] and more accurate in identifying and removing involved nodes than SNB alone [24, 25]. If TAD can safely and effectively replace ANC for patients with low-volume nodal disease having primary surgery, it would reduce patient morbidity, whilst continuing to provide accurate staging information which is important for prognostication and to inform appropriate selection of adjuvant therapies. TAD is already being used in the neoadjuvant setting in many units. UK Surgeons, therefore, have the necessary experience to be able to perform the procedure in the primary surgical setting in a future trial. The variation in TAD techniques identified by the survey, however, highlights the need for careful surgical quality assurance within the trial to agree the prohibited and mandatory components of the technique so that TAD can be standardised and delivered in consistent way across participating centres within the study [26].
Other studies evaluating TAD for node-positive patients having primary surgery are ongoing and include TADEN (NCT04671511); a Canadian prospective cohort study assessing the technical feasibility of TAD and the international TAXIS RCT (NCT03513614) [27]. TAXIS is comparing tailored axillary surgery (TAS) in combination with axillary radiotherapy with ANC in node-positive patients who have residual disease after neoadjuvant chemotherapy in addition to those having primary surgery. In TAXIS, all patients receiving more limited surgery will also receive nodal radiotherapy. As a future UK trial would aim to only include patients with low-volume nodal disease, the addition of radiotherapy is likely to represent overtreatment in this group so a trial specifically comparing the outcomes of TAD alone vs ANC is needed in the UK to change practice.
This national practice survey has informed the feasibility and design of a future axillary de-escalation study, but it has limitations. First, it only includes the views of a third of UK breast units so may not be representative of the views of the breast cancer community as a whole. Responses were, however, received from units of various sizes across the UK, the majority of whom felt that there was uncertainty regarding management of patients with low-volume nodal disease having primary surgery. Furthermore, over 30 units expressed an interest in participating in a future trial suggesting that effective recruitment to a future trial would be feasible. It is possible that unit practices may differ from those reported in the survey or that the survey reflected the views of the completing clinician, rather than the MDT as a whole. Centres may also have overestimated the numbers of potentially eligible patients that they see every year, but numbers are broadly consistent with known rates of nodal positivity at presentation, suggesting this is unlikely. This survey has, therefore, provided valuable information regarding the enthusiasm for and design of a future trial comparing TAD and ANC in the UK.
TAD may be a safe and effective alternative to ANC in patients with low-volume axillary nodal disease having primary surgery, but robust evaluation is necessary to prevent haphazard adoption of TAD and potential patient harm. UK practice is evolving rapidly as 15% of units surveyed have already adopted TAD in this setting, despite the absence of high-quality evidence to support a change in practice. De-escalation of treatment in the absence of robust confirmatory evidence risks compromising the significant progress made in effective treatments to improve breast cancer outcomes as a consequence of research over the last few decades. This survey suggests that a future RCT comparing TAD and ANC in the UK may be feasible and has provided insights into key elements of trial design including potential inclusion criteria and outcome selection, in addition to highlighting the need for surgical quality assurance to standardise the technique. In the process of designing any trial, comprehensive patient and public involvement is also essential to ensure that the trial evaluates outcomes of importance to patients as well as professionals. This will optimise the trial success and allow the top UK research priority in breast cancer surgery to be effectively addressed.
As a result of the feasibility data reported here and further extensive patient and public involvement work with Independent Cancer Patients Voice (ICPV) [28], TADPOLE: A multicentre, pragmatic, phase III randomised controlled trial comparing Targeted Axillary Dissection vs axillary node clearance in patients with POsitive axillary Lymph nodes in Early breast cancer, has been designed, and is currently at the final stages of consideration for funding by the National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA) programme and will open to recruitment in 2025.
Data availability
The datasets generated and analysed during this study are stored under the provisions of the National Data Protection Act and the University of Bristol requirements. Data may be made available to bona fida researchers only, on reasonable request to the corresponding author, after their host institution has signed a Data Access Agreement.
References
Cancer Research UK (Cruk). https://www.cancerresearchuk.org/health-Professional/Cancer-Statistics/Statistics-By-Cancer-Type/Breast-Cancer. Accessed 20 Jul 2023
National Institute for Health and Care Excellence (Nice) (2018) Early and locally advanced breast cancer: diagnosis and management [Ng101]
Disipio T et al (2013) Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol 14(6):500–515
Wang L et al (2016) Predictors of persistent pain after breast cancer surgery: a systematic review and meta-analysis of observational studies. CMAJ 188(14):E352–E361
Giuliano AE et al (2016) Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (Alliance) Acosog Z0011 randomized trial. Ann Surg 264(3):413–420
Giuliano AE et al (2017) Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the Acosog Z0011 (Alliance) randomized clinical trial. JAMA 318(10):918–926
Giuliano AE, Chung AP (2010) Long-term follow-up confirms the oncologic safety of sentinel node biopsy without axillary dissection in node-negative breast cancer patients. Ann Surg 251(4):601–603
Giuliano AE et al (2011) Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305(6):569–575
Giuliano AE et al (2010) Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg 252(3): 426–432;discussion 432–433
Galimberti V et al (2018) Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (Ibcsg 23–01): 10-year follow-up of a randomised, controlled phase 3 trial. Lancet Oncol 19(10):1385–1393
Galimberti V et al (2013) Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (Ibcsg 23–01): a phase 3 randomised controlled trial. Lancet Oncol 14(4):297–305
Heiranizadeh N et al (2022) Comparing early-stage breast cancer patients with sentinel lymph node metastasis with and without completion axillary lymph node dissection: a systematic review and meta-analysis. Asian Pac J Cancer Prev 23(8):2561–2571
Huang TW, Su CM, Tam KW (2021) Axillary management in women with early breast cancer and limited sentinel node metastasis: a systematic review and metaanalysis of real-world evidence in the post-Acosog Z0011 era. Ann Surg Oncol 28(2):920–929
Gou Z et al (2022) Trends in axillary surgery and clinical outcomes among breast cancer patients with sentinel node metastasis. Breast 63:9–15
Potter S et al (2023) Identifying research priorities in breast cancer surgery: a UK priority setting partnership with the James Lind Alliance. Breast Cancer Res Treat 197(1):39–49
Goyal A et al (2006) Factors affecting failed localisation and false-negative rates of sentinel node biopsy in breast cancer-results of the almanac validation phase. Breast Cancer Res Treat 99(2):203–208
Aragon-Sanchez S et al (2022) Feasibility of targeted axillary dissection for de-escalation of surgical treatment after neoadjuvant chemotherapy in breast cancer. Surg Oncol 44:101823
Bhattacharya I, Doughty J, Irvine T, Makris A, Palmieri C, Pinder S, Roche N, Vinnicombe S (2023) Neoadjuvant chemotherapy: multidisciplinary guidance.
Lee J et al (2023) Ten-year oncologic outcomes in T1–3n1 breast cancer after targeted axillary sampling: a retrospective study. Ann Surg Oncol 30(8):4669–4677
Macneill F, Irvine T (2021) Breast Surgery, Girft Programme National Specialty Report
Tseng J et al (2021) Changes in utilization of axillary dissection in women with invasive breast cancer and sentinel node metastasis after the Acosog Z0011 trial. Breast J 27(3):216–221
Poodt IGM et al (2018) Trends on axillary surgery in nondistant metastatic breast cancer patients treated between 2011 and 2015: a Dutch population-based study in the Acosog-Z0011 and Amaros era. Ann Surg 268(6):1084–1090
Goyal A et al (2021) Posnoc-positive sentinel node: adjuvant therapy alone versus adjuvant therapy plus clearance or axillary radiotherapy: a randomised controlled trial of axillary treatment in women with early-stage breast cancer who have metastases in one or two sentinel nodes. BMJ Open 11(12):E054365
Boughey JC et al (2016) Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0–T4, N1–N2) who receive neoadjuvant chemotherapy: results from Acosog Z1071 (Alliance). Ann Surg 263(4):802–807
Swarnkar PK et al (2021) The evolving role of marked lymph node biopsy (MLNB) and targeted axillary dissection (TAD) after neoadjuvant chemotherapy (NACT) for node-positive breast cancer: systematic review and pooled analysis. Cancers (Basel) 13(7):1539
Blencowe NS et al (2016) Standardizing and monitoring the delivery of surgical interventions in randomized clinical trials. Br J Surg 103(10):1377–1384
Henke G et al (2018) Tailored axillary surgery with or without axillary lymph node dissection followed by radiotherapy in patients with clinically node-positive breast cancer (taxis): study protocol for a multicenter, randomized phase-III trial. Trials 19(1):667
Independent Cancer Patient Voice (ICPV) https://Www.Independentcancerpatientsvoice.Org.Uk. Accessed 6 Mar 2023
Funding
This work was supported by an NIHR Academic Clinical Lectureship (CL-2020-25-002) for Katherine Fairhurst. Shelley Potter is an NIHR Clinician Scientist (CS-2016-16-019). The views expressed are those of the authors and not necessarily those of the UK National Health Service or National Institute for Health and Care Research.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to study conception and design. Material preparation and data collection were performed by Katherine Fairhurst. Data analysis was completed by Katherine Fairhurst. The first draft of the manuscript was written by Katherine Fairhurst and all authors read and approved the final manuscript. The list of collaborators are trainees and consultants who provided useable data for the survey and provided their name for publication as part of survey completion.
Corresponding author
Ethics declarations
Conflict of interest
SMcI reports honoraria from MSD, Roche, BD, Lilly, Novartis and Astra Zeneca, conference travel and support from Roche, Lilly and MSD, and institutional research funding from Novartis. RIC declares institutional research support from SECA and Astra Zeneca. KF and SP have no conflicts of interest to declare.
Ethical approval
Not required.
Consent to participate
All participants voluntarily participated and were made aware of potential publication. All data is presented anonymously, and no patient participants were involved.
Previous communication
Abstract (Poster at Association of Breast Surgery Conference): Fairhurst, Katherine et al. Targeted axillary dissection in low volume nodal disease at diagnosis: UK practice survey (TADPOLE), European Journal of Surgical Oncology, Volume 45, Issue 9, e249.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fairhurst, K., McIntosh, S.A., Cutress, R.I. et al. Current axillary management of patients with early breast cancer and low-volume nodal disease undergoing primary surgery: results of a United Kingdom national practice survey. Breast Cancer Res Treat (2024). https://doi.org/10.1007/s10549-024-07328-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10549-024-07328-4