Abstract
Purpose
Beta blockers (BBs) are commonly used cardiovascular medications, and their association with breast cancer outcomes has been examined in several previous observational studies and meta-analyses. In this study, an updated meta-analysis was undertaken to ascertain the association between BBs and both breast cancer death (BCD) and breast cancer recurrence (BCR).
Methods
Articles were sourced from various databases up until the 14th of August 2023. Effect estimates were pooled using the random effects model, and the Higgins I2 statistic was computed to ascertain heterogeneity. Subgroup analyses were conducted by the potential for immortal time bias (ITB), the exposure period (prediagnosis vs postdiagnosis), and type of BB (selective vs non-selective). Publication bias was assessed using funnel plots and Egger’s regression tests.
Results
Twenty-four studies were included. Pooled results showed that there was no statistically significant association between BB use and both BCD (19 studies, hazard ratio = 0.90, 95% CI 0.78–1.04) and BCR (16 studies, HR = 0.87, 95% CI 0.71–1.08). After removing studies with ITB, the associations were attenuated towards the null. There was no effect modification for either outcome when stratifying by the exposure period or type of BB. There was clear evidence of publication bias for both outcomes.
Conclusion
In this meta-analysis, we found no evidence of an association between BB use and both BCD and BCR. Removing studies with ITB attenuated the associations towards the null, but there was no effect modification by the exposure period or type of BB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cancer in women and the leading cause of female cancer mortality worldwide [1]. Comorbidities are common in patients with breast cancer [2], and there is a high and increasing prevalence of risk factors for both breast cancer and ischemic heart disease among Western women [3,4,5]. As such, many patients with breast cancer use prescribed medications for cardiovascular conditions. Ascertaining the association between commonly used cardiovascular medications and breast cancer outcomes is therefore warranted. Beta blockers (BBs), principally indicated for angina, arrhythmias, heart failure, hypertension, and myocardial infarction [6, 7], are commonly used cardiovascular medications that have been used in Western medicine for decades [8,9,10].
Human breast cancer cells have beta-adrenergic receptors [11]. Responses induced by beta-adrenergic signalling include upregulated expression of metastasis-associated genes involved in inflammation, angiogenesis, and tissue invasion, and downregulated expression of genes facilitating anti-tumour immune responses [12]. Beta-adrenergic receptors mediate the catecholamine hormones produced in the stress response, which in turn produce the aforementioned responses [13]. These hormones can be blocked by BBs, resulting in a potential protective effect through interference with tumour cell proliferation and migration, as well as tumoural angiogenesis [14, 15]. On the basis of this evidence, a number of observational studies have been carried out, and several meta-analyses have been published to summarise these studies [16,17,18,19,20,21,22]. Published between 2015 and 2023 and covering studies published up until the 1st of January 2023, most of these meta-analyses have shown no statistically significant association between the use of BBs and the prognosis of breast cancer.
In the meta-analyses published to date, some of the more recent observational studies have not been included [23, 24]. Further, there have not been many meta-analyses that have examined potentially important effect modifiers of the association such as the presence of immortal time bias (ITB), the type of BB (selective vs non-selective), or the exposure period in which BBs were used (prediagnosis vs postdiagnosis). ITB, for example, is a bias in which a spurious survival advantage is invariably conferred to the user group in studies that count user time in a period where events could not occur by design [25]. Further, several preclinical studies have suggested that non-selective BBs may have a higher efficacy in inhibiting pathways involved in breast cancer progression and metastasis [15, 26]. It has also been suggested that BBs taken prior to breast cancer diagnosis (and around the time of diagnosis) may be more efficacious in preventing breast cancer progression/metastases than BBs taken after diagnosis [15, 27,28,29,30,31,32,33]. As such, the objective of the current paper was to perform an updated meta-analysis examining the association between BBs and both breast cancer death (BCD) and breast cancer recurrence (BCR), and also examine whether the associations differ across potentially important subgroups.
Methods
Literature search/data sources
PubMed, Embase, Medline, Web of Science, Google Scholar, and the Cochrane Library databases were searched using the keywords [beta, beta blocker, beta antagonist, metoprolol, propranolol, carvedilol, labetalol, celiprolol, acebutolol, bisoprolol, breast cancer, breast carcinoma, cancer, carcinoma, recurrence, metastasis, outcome, death, prognosis, survival, mortality, proliferation] in combinations of ‘AND’ or ‘OR’. There was no restriction placed on the earliest date of publication, but articles were only selected for review if they analysed human breast cancer patients and were written in English. Further articles were sourced from scanning the reference lists of individual observational studies and meta-analyses found using the aforementioned search criteria. Literature was sourced up until the 14th of August 2023.
Study selection/eligibility criteria
Studies were eligible for inclusion if they met the following criteria: (1) Included women with breast cancer as the cohort of interest; (2) Analysed data from cohort studies, case–control studies, or randomised controlled trials (including retrospective analyses of already published RCTs); (3) Assessed BB use as the exposure of interest (with no minimum dose requirement); (4) Reported BCD or BCR (or both) as the outcome(s) of interest; (5) Reported risk estimates and 95% confidence intervals for the associations of interest; and (6) Were published as full length articles. We did not consider systematic reviews, laboratory experiments, case reports, ecological studies, conference abstracts, or editorials for inclusion in this meta-analysis.
Data extraction
The titles and abstracts of each individual study were firstly examined to assess their eligibility, and relevant information from each study was extracted. Information extracted from each study included the first author and year of study, country of study, the number of women under study, study design, patient characteristics (such as cancer stage and age), exposure definition (pre or postdiagnosis), the source of exposure data, median or mean follow-up time, the potential for ITB (studies were judged to be susceptible to ITB when they used a ‘time fixed’ postdiagnosis definition of medication use), covariates adjusted for, the types of BBs used by women (e.g., all BBs, selective BBs, non-selective BBs, or a combination), and the reference group used (e.g. BB users vs all BB non-users or BB users vs a different comparison group). When studies used a stepwise approach for covariate adjustment, the fully adjusted HR was taken.
Statistical analysis
The main analysis assessed the association between BB use at any time and breast cancer outcomes, pooling the data from all included studies. When studies reported risk estimates for BBs used both before and after breast cancer diagnosis for the same cohort, only the risk estimates for postdiagnosis use were taken (to avoid sample overlap), as it can be considered that postdiagnosis use of medications over the course of breast cancer therapy is the more clinically relevant exposure period. Risk estimates and 95% confidence intervals were transformed onto the log scale, as recommended [34]. To pool risk estimates into a summary estimate, the inverse variance method with random effects model was used. We used a random effects model because it seems plausible that the effect of BBs could vary from study to study due to factors other than sampling variability [35]. In the paper by Lorona and others [36], the HR for the association between BB use and both BCD and BCR was initially stratified by breast cancer molecular subtype (luminal, triple negative, and HER2 positive), and no overall HR was reported. These HRs were firstly pooled using a random effects model to derive an overall summary estimate for the association among all types of breast cancer, and the resulting pooled HR was then included in this meta-analysis. A similar method was used to pool results for the association between prediagnosis BB use and BCR in the paper by Sorensen and colleagues [37], in which the HR for prediagnosis use was initially stratified by the number of prescribed tablets. The Higgins I2 statistic was computed for each pooled estimate to ascertain the amount of heterogeneity present between studies, and an I2 statistic of > 50% indicated that there was a significant amount of heterogeneity [38].
To explore reasons for heterogeneity between studies and to assess the impact of different potential modifying variables on the associations of interest, subgroup analyses [39] were carried out by the potential for ITB (yes vs no), the exposure period in which BBs were used (prediagnosis vs postdiagnosis), and the type of BB used (selective vs non-selective). Because of the bias inherent in studies judged to be susceptible to ITB [25], the Subgroup analyses by exposure period and type of BB used were restricted to studies judged not to be susceptible to ITB. Studies were excluded in the subgroup analysis by exposure period if they assessed BB use both before and after diagnosis in the same analysis. To formally test for differences between subgroups, a random effects meta regression was used [40]. Sensitivity analyses were conducted to iteratively assess the impact of excluding each individual study on the overall summary estimate [41]. Lastly, funnel plots [42] were generated, and Egger’s regression tests [43] were performed to assess the presence of any potential publication bias. All reported p-values are two sided and were considered statistically significant if p < 0.05. All analyses were conducted in STATA 17.0 (StataCorp, College Station, TX).
Results
Literature search
A total of 489 studies (after removing duplicates) were retrieved from the selected databases during the literature search and included for initial screening (Fig. 1). 132 reports were assessed in full, and after making various exclusions, 24 studies were deemed to meet the inclusion criteria and were included in this meta-analysis [23, 24, 36, 37, 44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63]. The detailed characteristics of these 24 studies are shown in Table 1.
Study characteristics
Overall, this meta-analysis included 24 studies [23, 24, 36, 37, 44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63] with a combined total of 253,082 women with breast cancer. Nineteen of these studies assessed BCD as an outcome, while sixteen assessed BCR as an outcome (eight of these studies assessed both outcomes). There were three studies that included two different cohorts/datasets, these being the studies by Spera et al. [63] (in which they retrospectively analysed two different clinical trials), Barron et al. [45] (in which they analysed propranolol and atenolol in two separate cohorts), and Chang et al. [23] (in which cohorts from both Italy and Norway were analysed). As such, there were 27 unique datasets available for this meta-analysis. Eleven of the 24 studies were conducted in Europe (including the UK), ten in North America, and three were from the Asia/Oceania region. Sixteen were designed as retrospective cohort studies, six were prospective cohort studies, one was a nested case control study, and one retrospectively analysed two clinical trials. Almost all studies (except one [62]) adjusted for covariates, and every study that adjusted for confounders adjusted for age at the very least. In general, a range of confounders were usually adjusted for, including demographic variables, breast cancer clinical variables, comorbidities, and concomitant medication usage. For example, potentially important confounders such as cancer stage, comorbidities, and concomitant medication use were adjusted for in 17/24, 11/24, and 15/24 studies respectively. The mean or median follow-up time across the 24 studies ranged from 2.1 to 10.5 years, with ten studies reporting a mean/median follow-up time of 0–5 years, nine reporting a mean/median follow-up time of 5–10 years, and two reporting a mean/median follow-up time of 10 + years. Follow-up time was not reported in three of the studies. Seventeen studies analysed BB use after the diagnosis of breast cancer, six considered prediagnosis BB use as the exposure of interest, and one considered BB use both before and after diagnosis in the same analysis. Twelve studies ascertained medication use through prescription records, five through medical records, one through self-reported patient data, and six through a combination of data sources. Twenty studies were judged not to be susceptible to ITB, while four were. Finally, there were eleven studies that stratified BB use by type (selective vs non-selective), while thirteen did not.
Association between BB use and breast cancer related deaths
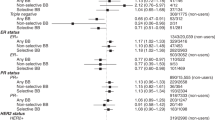
Overall, 17 studies (with two of these studies contributing two datasets each) were included in the meta-analysis examining the association between BB use and breast cancer specific death. Pooled results with a random effects model showed that there was no statistically significant association between BB use and BCD, with only a small reduction in the HR (HR = 0.90, 95% CI 0.78–1.04, p = 0.16; Fig. 2a). There was significant heterogeneity between these studies (I2 = 84.42%). Sensitivity analysis iteratively recalculating the HR by removing one study at a time did not significantly alter the results (HRs 0.86–0.96, all p > 0.05; Supplementary Fig. 1A). Further, the confidence intervals of all the meta-analyses while removing one study at a time included the HR of the overall summary estimate. Subgroup analyses showed that the presence of ITB may affect the outcome. In the studies judged to be susceptible to this bias, the results indicated a statistically significant protective association between BB use and BCD (HR = 0.42, 95% CI 0.25–0.71, p = 0.001, Table 2), but there was no association in the studies judged not to be susceptible (HR = 0.99, 95% CI 0.91–1.09, p = 0.89; p for subgroup difference = 0.002). Other analyses revealed that stratifying the association by other potential modifiers (exposure period and type of BB used) did not significantly change the results (p-values for subgroup differences both > 0.05).
Association between BB use and breast cancer recurrence
Overall, 15 studies (with one of these studies contributing two datasets) were included in the meta-analysis examining the association between BB use and breast cancer recurrence. Pooled results with a random effects model showed that there was no statistically significant association between BB use and BCR, with only a small reduction in the HR (HR = 0.87, 95% CI 0.71–1.08, p = 0.22; Fig. 2b). There was significant heterogeneity between these studies (I2 = 87.09%). Sensitivity analysis iteratively recalculating the HR by removing one study at a time did not significantly alter the results (HRs 0.84–0.90, all p > 0.05; Supplementary Fig. 1B). Further, the confidence intervals of all the meta-analyses while removing one study at a time included the HR of the overall summary estimate. Subgroup analyses showed that the presence of immortal time bias may affect the outcome. In the studies judged to be susceptible to this bias, the results indicated a statistically significant protective association between BB use and BCR (HR = 0.67, 95% CI 0.53–0.84, p = 0.001, Table 2), but there was no association in the studies judged not to be susceptible (HR = 1.02, 95% CI 0.80–1.29, p = 0.88; p for subgroup difference = 0.02). Other analyses revealed that stratifying the association by other potential modifiers (exposure period and type of BB used) did not significantly change the results (p-values for subgroup differences both > 0.05).
Publication bias
Funnel plots for the association between BB use and both BCD and BCR showed clear evidence of asymmetry and publication bias (Figs. 3a and 3b). In the plot with BCR as the outcome, there was asymmetry in terms of the number of studies on either side of the average effect size, with the plot having more studies on the left-hand side (meaning that there were more studies that showed a lower HR than the overall average effect size relative to studies that showed a higher HR). Further, there was very clear evidence of small study effects in both plots, with big clusters of small studies with very large effect sizes in the bottom left of the plots. The results of Egger’s regression tests for these outcomes were in concordance with a visual inspection of the respective funnel plots (p-values for small study effects < 0.05 in both plots).
Discussion
In this meta-analysis, we found no statistically significant association between BB use and both BCD and BCR, with only a small reduction in the HR for both outcomes. The results were similar when leaving one study out at a time for each outcome, suggesting that the results are relatively stable and were not affected by the presence of outliers. These findings are consistent with some of the contemporary meta-analyses examining the association between BB use and breast cancer prognosis [18,19,20,21,22], which all found a small reduction in the HR for both BCD and BCR, although none of these associations reached statistical significance either. One recently published meta-analysis also found no association between BB use and BCD, but a small and statistically significant increased risk for BCR [65]. Two older meta-analyses [16, 17] both found a statistically significant protective association for BCD and a non-statistically significant risk reduction for BCR, however these meta-analyses included far fewer studies than both the current and more recently published meta-analyses. Furthermore, older meta-analyses were more influenced by older observational studies that were often prone to methodological limitations such as immortal time bias [25]. For example, the summary estimate for BCD in the meta-analysis by Childers and others [16] only included four studies, one of which was judged to be susceptible to ITB [44] in the current meta-analysis. The summary estimate for BCD in the meta-analysis by Raimondi and others [17] included the same studies as the meta-analysis by Childers et al., plus two additional studies, one of which was judged to be susceptible to ITB [49] in the current meta-analysis.
Immortal time bias (ITB) is a bias introduced in pharmacoepidemiological studies in which a survival advantage is conferred to the user group by way of misattributing user time over a period where events could not occur by design [25]. For example, if a postdiagnostic exposure period is used and a binary yes/no indicator is used to model medication use (i.e., a time fixed approach), the time between cancer diagnosis and the first medication dispensing is ‘immortal’ because the patient needed to have survived this period to be dispensed a medication. Lévesque and colleagues showed that medications with no biological basis for doing so (e.g., NSAIDS) could be made to show a decreased risk of diabetes progression by modelling medication use through a time fixed approach [66]. The subgroup analysis in this meta-analysis showed a similar (and expected) pattern, with statistically significant subgroup differences between studies judged to be susceptible to ITB relative to studies judged not to be susceptible to ITB for both outcomes. In fact, there was no longer a suggestion of a protective association for either outcome when studies judged to be susceptible to ITB were excluded from the analysis. Only one previous meta-analysis in this area has examined ITB as a potential effect modifier [19], and it found no suggestion of effect modification. The pooled HR with BCR as the outcome in this meta-analysis was 0.88 (0.66–1.17), and 0.83 (0.54–1.30) when removing studies judged to be susceptible to ITB. There were some disagreements between this meta-analysis and the current meta-analysis in terms of judging studies to be prone to be ITB or not. In the current meta-analysis, the studies by Boudreau et al. [60] and Ganz et al. [46] were judged not to be susceptible to ITB, while the study by Powe et al. [44] was. However, the other meta-analysis [19] judged the studies by Boudreau et al. and Ganz et al. to be prone to ITB, and the study by Powe et al. to not be prone to ITB. In the current meta-analysis, we made a strong effort to correctly classify studies in terms of their risk of ITB, including emailing authors to clarify their BB exposure definition.
There have been hypotheses that BBs used prior to diagnosis (or around the time of diagnosis) may be more efficacious in inhibiting breast cancer progression than BBs used after diagnosis, as this time is generally very stressful for the patient and BBs can aid in alleviating this stress [15, 27,28,29,30,31,32,33]. Furthermore, there may be a synergistic and beneficial interaction between BBs and chemotherapy, as stress is conjectured to inhibit the effectiveness of chemotherapy through β-adrenergic signalling [71,72,73]. Post curative surgery, however, the antimetastatic effects of BBs may be minimal relative to the time period around diagnosis. Despite a number of studies in each subgroup for both outcomes, our meta-analysis was unable to ascertain a difference between the two exposure periods. One recently published meta-analysis [20] found a suggestion of a more protective association for pre diagnosis use with BCR as the outcome (p for subgroup difference = 0.09), however the number of studies in each subgroup was small relative to the current meta-analysis. Furthermore, this meta-analysis did not exclude studies judged to be susceptible to ITB in their subgroup analyses.
In the subgroup analysis by type of BB, there was no specificity shown in terms of either type (selective or non-selective) being more efficacious than the other. Several preclinical studies have suggested that non-selective BBs may have a higher efficacy in inhibiting pathways involved in breast cancer progression and metastasis [15, 26]. In particular, propranolol has been shown to have antimigratory and antiangiogenic properties in both animal models and human cancer cell lines [67,68,69,70,71]. Despite this, the subgroup results by type were not suggestive of a differential effect, with nonsignificant subgroup differences found for both outcomes. A somewhat recently published meta-analysis also found little evidence of effect modification by type [21], however it is worthy to note that the number of studies included in this subgroup analysis were small for this meta-analysis as well the current meta-analysis. In the current meta-analysis, there were only four studies in each group for the recurrence outcome, which likely contributed to relatively unstable estimates.
It must be acknowledged that there was a significant amount of between study heterogeneity present in this meta-analysis, even in many of the individual arms of the subgroup analyses. As shown in Table 1, different studies were often conducted over different time periods and in different countries, inevitably meaning that BBs were often used in different clinical contexts. BBs were commonly indicated for hypertension 15–20 years ago, however heart failure is now their most common indication [9, 74]. As such, BB users are likely to be less healthy in contemporary studies relative to older studies. There was also little uniformity regarding the characteristics of women enrolled or analysed, with a range of different cancer stages and ages studied. Similarly, the covariates adjusted for varied widely across studies, with some studies making comprehensive adjustments for a range of covariates, while other studies likely lacked access to a similar range of potential confounders. Finally, even though the prediagnosis and postdiagnosis exposure periods were examined in subgroup analyses, there was also heterogeneity present within these groups. For example, postdiagnosis BB use may mean use in the first year after diagnosis only or use any time after diagnosis. All of these factors likely play a role in influencing any effect estimate derived, especially when > 1 of these factors differ from study to study.
We found clear evidence of publication bias and small study effects, with evidence of asymmetry in the funnel plot with BCR as the outcome and big clusters of small studies with very large effect sizes for both outcomes. In the main summary estimates, there was only a suggestion of a protective association for BBs, with non-statistically significant results for both outcomes. If publication bias was not present in this meta-analysis and the most imprecise studies with very large and protective effect sizes were omitted, it would mean that the overall HR would attenuate towards the null (1.0) for both outcomes. This would mean that we would have even less confidence in a potential protective effect for BBs, and along with the ITB subgroup analysis described above, gives credence to the hypothesis that BBs have little or no effect on the prognosis of breast cancer.
The main strength of this meta-analysis is the large number of observational studies included and the resulting total number of women with breast cancer analysed (253,082). Furthermore, we studied both BCD and BCR as outcomes, and the consistency in the results between these outcomes gives us confidence in the interpretation of the meta-analysis. We also conducted subgroup analyses by ITB, exposure period, and the type of BB used, which were all subgroups we were interested in a priori with legitimate methodological or clinical justifications for analysing.
This study is not without its limitations. As mentioned above, there was significant between study heterogeneity in this meta-analysis, and as a result, it is difficult to determine if the pooled effect estimates derived are driven by BB use exclusively, extraneous factors such as the study period and/or other modifying factors between studies, or a combination of these. Furthermore, even though preclinical evidence suggests that the effect of BBs may vary by subtype (and might be efficacious for triple negative breast cancer in particular) [33, 75, 76], we did not conduct a subgroup analysis by breast cancer type due to how rarely this was reported. Finally, although there have been suggestions that long term BB use may be beneficial in preventing the growth and/or spread of breast cancer [45, 58], we were unable to conduct a subgroup analysis by dose of BB due to how rarely this was reported as well.
In conclusion, we found no evidence of BBs being protective or increasing risk for the outcomes of BCD and BCR in this meta-analysis. Protective effects reported in some previous meta-analyses are likely due to studies with immortal time bias, and also to publication bias. We found no indication of effect modification by either the type of BB used (selective vs non-selective) or the exposure period in which BBs were used (prediagnosis vs postdiagnosis). However, our findings are based on observational studies and should be confirmed in future clinical trials. Such work should ideally focus on subgroups for which preclinical evidence of potential benefit has been found, such as specific types of BBs (e.g., non-selective) or subtypes of breast cancer (e.g., triple negative).
Data availability
Not applicable.
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Land LH, Dalton SO, Jensen MB et al (2012) Influence of comorbidity on the effect of adjuvant treatment and age in patients with early-stage breast cancer. Br J Cancer 107:1901–1907. https://doi.org/10.1038/bjc.2012.472
Mehta LS, Watson KE, Barac A et al (2018) Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation 137:e30–e66
Ministry of Health. Annual update of key results 2018/19: New Zealand Health Survey. https://www.health.govt.nz/publication/annual-update-key-results-2018-19-new-zealand-health-survey (Date Accessed 2019)
Ford ES, Li C, Zhao G et al (2010) Trends in low-risk lifestyle factors among adults in the United States: findings from the behavioral risk factor surveillance system 1996–2007. Prev Med 51:403–407. https://doi.org/10.1016/j.ypmed.2010.08.002
Ram CV (2010) Beta-blockers in hypertension. Am J Cardiol 106:1819–1825. https://doi.org/10.1016/j.amjcard.2010.08.023
Aronow WS (2010) Current role of beta-blockers in the treatment of hypertension. Expert Opin Pharmacother 11:2599–2607. https://doi.org/10.1517/14656566.2010.482561
Jackson R, Barham P, Bills J et al (1993) Management of raised blood pressure in New Zealand: a discussion document. BMJ 307:107–110. https://doi.org/10.1136/bmj.307.6896.107
Best Practice Advocacy Centre New Zealand. Beta-blockers for cardiovascular conditions: one size does not fit all patients. https://bpac.org.nz/2017/beta-blockers.aspx (Date Accessed 2017)
Bangalore S, Messerli FH, Kostis JB et al (2007) Cardiovascular protection using beta-blockers: a critical review of the evidence. J Am Coll Cardiol 50:563–572. https://doi.org/10.1016/j.jacc.2007.04.060
Vandewalle B, Revillion F, Lefebvre J (1990) Functional beta-adrenergic receptors in breast cancer cells. J Cancer Res Clin Oncol 116:303–306
Cole SW, Sood AK (2012) Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res 18:1201–1206. https://doi.org/10.1158/1078-0432.CCR-11-0641
Tilan J, Kitlinska J (2010) Sympathetic neurotransmitters and tumor angiogenesis-link between stress and cancer progression. J Oncol 2010:539706. https://doi.org/10.1155/2010/539706
Vaklavas C, Chatzizisis YS, Tsimberidou AM (2011) Common cardiovascular medications in cancer therapeutics. Pharmacol Ther 130:177–190. https://doi.org/10.1016/j.pharmthera.2011.01.009
Sloan EK, Priceman SJ, Cox BF et al (2010) The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res 70:7042–7052. https://doi.org/10.1158/0008-5472.CAN-10-0522
Childers WK, Hollenbeak CS, Cheriyath P (2015) Beta-blockers reduce breast cancer recurrence and breast cancer death: a meta-analysis. Clin Breast Cancer 15:426–431. https://doi.org/10.1016/j.clbc.2015.07.001
Raimondi S, Botteri E, Munzone E et al (2016) Use of beta-blockers, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers and breast cancer survival: systematic review and meta-analysis. Int J Cancer 139:212–219. https://doi.org/10.1002/ijc.30062
Kim HY, Jung YJ, Lee SH et al (2017) Is beta-blocker use beneficial in breast cancer? A meta-analysis. Oncology 92:264–268. https://doi.org/10.1159/000455143
Yap A, Lopez-Olivo MA, Dubowitz J et al (2018) Effect of beta-blockers on cancer recurrence and survival: a meta-analysis of epidemiological and perioperative studies. Br J Anaesth 121:45–57. https://doi.org/10.1016/j.bja.2018.03.024
Li C, Li T, Tang R et al (2020) β-blocker use is not associated with improved clinical outcomes in women with breast cancer: a meta-analysis. Biosci Rep. https://doi.org/10.1042/BSR20200721
Caparica R, Bruzzone M, Agostinetto E et al (2021) Beta-blockers in early-stage breast cancer: a systematic review and meta-analysis. ESMO Open 6:100066. https://doi.org/10.1016/j.esmoop.2021.100066
Xie Y, Wang M, Xu P et al (2021) Association between antihypertensive medication use and breast cancer: a systematic review and meta-analysis. Front Pharmacol 12:609901. https://doi.org/10.3389/fphar.2021.609901
Chang A, Botteri E, Gillis RD et al (2023) Beta-blockade enhances anthracycline control of metastasis in triple-negative breast cancer. Sci Transl Med 15:1147. https://doi.org/10.1126/scitranslmed.adf1147
Hsieh HH, Wu TY, Chen CH et al (2023) Survival outcomes of beta-blocker usage in HER2-positive advanced breast cancer patients: a retrospective cohort study. Ther Adv Drug Saf 14:20420986231181336. https://doi.org/10.1177/20420986231181338
Suissa S (2008) Immortal time bias in pharmacoepidemiology. Am J Epidemiol 167:492–499
Lang K, Drell TLT, Lindecke A et al (2004) Induction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugs. Int J Cancer 112:231–238. https://doi.org/10.1002/ijc.20410
Le CP, Nowell CJ, Kim-Fuchs C et al (2016) Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun 7:10634. https://doi.org/10.1038/ncomms10634
Thaker PH, Han LY, Kamat AA et al (2006) Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 12:939–944. https://doi.org/10.1038/nm1447
Hiller JG, Cole SW, Crone EM et al (2020) Preoperative beta-blockade with propranolol reduces biomarkers of metastasis in breast cancer: a phase II randomized trial. Clin Cancer Res 26:1803–1811. https://doi.org/10.1158/1078-0432.CCR-19-2641
Hiller JG, Perry NJ, Poulogiannis G et al (2018) Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol 15:205–218. https://doi.org/10.1038/nrclinonc.2017.194
Creed SJ, Le CP, Hassan M et al (2015) β2-adrenoceptor signaling regulates invadopodia formation to enhance tumor cell invasion. Breast Cancer Res 17:145. https://doi.org/10.1186/s13058-015-0655-3
Kim TH, Gill NK, Nyberg KD et al (2016) Cancer cells become less deformable and more invasive with activation of beta-adrenergic signaling. J Cell Sci 129:4563–4575. https://doi.org/10.1242/jcs.194803
Bucsek MJ, Qiao G, MacDonald CR et al (2017) β-adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8+ T cells and undermines checkpoint inhibitor therapy. Cancer Res 77:5639–5651. https://doi.org/10.1158/0008-5472.CAN-17-0546
Cumpston M, Li T, Page MJ et al. (2019) Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 10: 142 doi:https://doi.org/10.1002/14651858.ED000142.
Borenstein M, Hedges LV, Higgins JP et al (2010) A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 1:97–111. https://doi.org/10.1002/jrsm.12
Lorona NC, Cook LS, Tang MC et al (2021) Antihypertensive medications and risks of recurrence and mortality in luminal, triple-negative, and HER2-overexpressing breast cancer. Cancer Causes Control 32:1375–1384. https://doi.org/10.1007/s10552-021-01485-3
Sørensen GV, Ganz PA, Cole SW et al (2013) Use of beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and risk of breast cancer recurrence: a Danish nationwide prospective cohort study. J Clin Oncol 31:2265–2272. https://doi.org/10.1200/JCO.2012.43.9190
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. https://doi.org/10.1136/bmj.327.7414.557
Borenstein M, Higgins JP (2013) Meta-analysis and subgroups. Prev Sci 14:134–143. https://doi.org/10.1007/s11121-013-0377-7
Baker WL, White CM, Cappelleri JC et al (2009) Understanding heterogeneity in meta-analysis: the role of meta-regression. Int J Clin Pract 63:1426–1434. https://doi.org/10.1111/j.1742-1241.2009.02168.x
Higgins JP (2008) Commentary: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol 37:1158–1160. https://doi.org/10.1093/ije/dyn204
Sterne JA, Becker BJ, Egger M (2005) The funnel plot. In: Rothstein HR, Sutton AJ, Borenstein MS (eds) Publication bias in meta-analysis: prevention, assessment and adjustments: Wiley. New York, pp 76–98
Harbord RM, Egger M, Sterne JA (2006) A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 25:3443–3457. https://doi.org/10.1002/sim.2380
Powe DG, Voss MJ, Zanker KS et al (2010) Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 1:628–638. https://doi.org/10.18632/oncotarget.101009
Barron TI, Connolly RM, Sharp L et al (2011) Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol 29:2635–2644. https://doi.org/10.1200/JCO.2010.33.5422
Ganz PA, Habel LA, Weltzien EK et al (2011) Examining the influence of beta blockers and ACE inhibitors on the risk for breast cancer recurrence: results from the LACE cohort. Breast Cancer Res Treat 129:549–556. https://doi.org/10.1007/s10549-011-1505-3
Botteri E, Munzone E, Rotmensz N et al (2013) Therapeutic effect of beta-blockers in triple-negative breast cancer postmenopausal women. Breast Cancer Res Treat 140:567–575. https://doi.org/10.1007/s10549-013-2654-3
Cardwell CR, Coleman HG, Murray LJ et al (2013) Beta-blocker usage and breast cancer survival: a nested case-control study within a UK clinical practice research datalink cohort. Int J Epidemiol 42:1852–1861. https://doi.org/10.1093/ije/dyt196
Chae YK, Brown EN, Lei X et al (2013) Use of ACE inhibitors and angiotensin receptor blockers and primary breast cancer outcomes. J Cancer 4:549–556. https://doi.org/10.7150/jca.6888
Holmes MD, Hankinson SE, Feskanich D et al (2013) Beta blockers and angiotensin-converting enzyme inhibitors’ purported benefit on breast cancer survival may be explained by aspirin use. Breast Cancer Res Treat 139:507–513. https://doi.org/10.1007/s10549-013-2553-7
Cardwell CR, Pottegard A, Vaes E et al (2016) Propranolol and survival from breast cancer: a pooled analysis of European breast cancer cohorts. Breast Cancer Res 18:119. https://doi.org/10.1186/s13058-016-0782-5
Chen L, Chubak J, Boudreau DM et al (2017) Use of antihypertensive medications and risk of adverse breast cancer outcomes in a SEER-medicare population. Cancer Epidemiol Biomark Prev 26:1603–1610. https://doi.org/10.1158/1055-9965.EPI-17-0346
Musselman RP, Bennett S, Li W et al (2018) Association between perioperative beta blocker use and cancer survival following surgical resection. Eur J Surg Oncol 44:1164–1169. https://doi.org/10.1016/j.ejso.2018.05.012
Cui Y, Wen W, Zheng T et al (2019) Use of antihypertensive medications and survival rates for breast, colorectal, lung, or stomach cancer. Am J Epidemiol 188:1512–1528. https://doi.org/10.1093/aje/kwz106
Santala EEE, Murto MO, Artama M et al (2020) Angiotensin receptor blockers associated with improved breast cancer survival-a nationwide cohort study from Finland. Cancer Epidemiol Biomark Prev 29:2376–2382. https://doi.org/10.1158/1055-9965.EPI-20-0711
Gillis RD, Botteri E, Chang A et al (2021) Carvedilol blocks neural regulation of breast cancer progression in vivo and is associated with reduced breast cancer mortality in patients. Eur J Cancer 147:106–116. https://doi.org/10.1016/j.ejca.2021.01.029
Løfling LL, Stoer NC, Sloan EK et al (2022) Beta-blockers and breast cancer survival by molecular subtypes: a population-based cohort study and meta-analysis. Br J Cancer 127:1086–1096. https://doi.org/10.1038/s41416-022-01891-7
Scott OW, Tin Tin S, Elwood JM et al (2022) Post-diagnostic beta blocker use and breast cancer-specific mortality: a population-based cohort study. Breast Cancer Res Treat 193:225–235. https://doi.org/10.1007/s10549-022-06528-0
Melhem-Bertrandt A, Chavez-Macgregor M, Lei X et al (2011) Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol 29:2645–2652. https://doi.org/10.1200/JCO.2010.33.4441
Boudreau DM, Yu O, Chubak J et al (2014) Comparative safety of cardiovascular medication use and breast cancer outcomes among women with early stage breast cancer. Breast Cancer Res Treat 144:405–416. https://doi.org/10.1007/s10549-014-2870-5
Sakellakis M, Kostaki A, Starakis I et al (2014) Beta-blocker use and risk of recurrence in patients with early breast cancer. Chemotherapy 60:288–289. https://doi.org/10.1159/000371871
Choy C, Raytis JL, Smith DD et al (2016) Inhibition of beta2-adrenergic receptor reduces triple-negative breast cancer brain metastases: the potential benefit of perioperative beta-blockade. Oncol Rep 35:3135–3142. https://doi.org/10.3892/or.2016.4710
Spera G, Fresco R, Fung H et al (2017) Beta blockers and improved progression-free survival in patients with advanced HER2 negative breast cancer: a retrospective analysis of the ROSE/TRIO-012 study. Ann Oncol 28:1836–1841. https://doi.org/10.1093/annonc/mdx264
Santala EE, Murto MO, Artama M et al (2020) Angiotensin receptor blockers associated with improved breast cancer survival: a nationwide cohort study from Finland. Cancer Epidemiol Biomark Prev 29:2376–2382
Yang J, Zhang S, Jiang W (2023) Impact of beta blockers on breast cancer incidence and prognosis. Clin Breast Cancer 23(664–671):e21. https://doi.org/10.1016/j.clbc.2023.05.014
Levesque LE, Hanley JA, Kezouh A et al (2010) Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 340:b5087. https://doi.org/10.1136/bmj.b5087
Annabi B, Lachambre MP, Plouffe K et al (2009) Propranolol adrenergic blockade inhibits human brain endothelial cells tubulogenesis and matrix metalloproteinase-9 secretion. Pharmacol Res 60:438–445. https://doi.org/10.1016/j.phrs.2009.05.005
Hajighasemi F, Hajighasemi S (2009) Effect of propranolol on angiogenic factors in human hematopoietic cell linesin vitro. Iran Biomed J 13:223–228
Lamy S, Lachambre MP, Lord-Dufour S et al (2010) Propranolol suppresses angiogenesis in vitro: inhibition of proliferation, migration, and differentiation of endothelial cells. Vascul Pharmacol 53:200–208. https://doi.org/10.1016/j.vph.2010.08.002
Yap TA, Sandhu SK, Workman P et al (2010) Envisioning the future of early anticancer drug development. Nat Rev Cancer 10:514–523. https://doi.org/10.1038/nrc2870
Pasquier E, Ciccolini J, Carre M et al (2011) Propranolol potentiates the anti-angiogenic effects and anti-tumor efficacy of chemotherapy agents: implication in breast cancer treatment. Oncotarget 2:797–809. https://doi.org/10.18632/oncotarget.343
Reeder A, Attar M, Nazario L et al (2015) Stress hormones reduce the efficacy of paclitaxel in triple negative breast cancer through induction of DNA damage. Br J Cancer 112:1461–1470. https://doi.org/10.1038/bjc.2015.133
Pasquier E, Street J, Pouchy C et al (2013) Beta-blockers increase response to chemotherapy via direct antitumour and anti-angiogenic mechanisms in neuroblastoma. Br J Cancer 108:2485–2494. https://doi.org/10.1038/bjc.2013.205
Wiysonge CS, Bradley HA, Volmink J et al (2017) Beta-blockers for hypertension. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD002003.pub5
Nissen MD, Sloan EK, Mattarollo SR (2018) beta-adrenergic signaling impairs antitumor CD8(+) T-cell responses to B-cell lymphoma immunotherapy. Cancer Immunol Res 6:98–109. https://doi.org/10.1158/2326-6066.CIR-17-0401
Kwa MJ, Adams S (2018) Checkpoint inhibitors in triple-negative breast cancer (TNBC): where to go from here. Cancer 124:2086–2103
Acknowledgements
We would like to thank the Auckland Medical Research Foundation for providing funding to carry out this study.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Oliver Scott was supported by an Auckland Medical Research Foundation doctoral scholarship (Ref: 1217004). This project was also supported by an Auckland Medical Research Foundation project grant (Ref: 1118017).
Author information
Authors and Affiliations
Contributions
OWS, STT, and JME substantially contributed to the conception and design of the study and interpreted the data alongside AC. OWS led the analysis and wrote the manuscript. All authors critically revised and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors have no relevant financial or non-financial interests to disclose.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Ethical approval.
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Scott, O.W., TinTin, S., Cavadino, A. et al. Beta-blocker use and breast cancer outcomes: a meta-analysis. Breast Cancer Res Treat 206, 443–463 (2024). https://doi.org/10.1007/s10549-024-07263-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-024-07263-4