Abstract
Background
Young patients with breast ductal carcinoma in situ (DCIS) often face a poorer prognosis. The genomic intricacies in young-onset DCIS, however, remain underexplored.
Methods
To address this gap, we undertook a comprehensive study encompassing exome, transcriptome, and vmethylome analyses. Our investigation included 20 DCIS samples (including 15 young-onset DCIS) and paired samples of normal breast tissue and blood.
Results
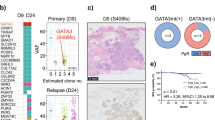
Through RNA sequencing, we identified two distinct DCIS subgroups: “immune hot” and “immune cold”. The “immune hot” subgroup was characterized by increased infiltration of lymphocytes and macrophages, elevated expression of PDCD1 and CTLA4, and reduced GATA3 expression. This group also exhibited active immunerelated transcriptional regulators. Mutational analysis revealed alterations in TP53 (38%), GATA3 (25%), and TTN (19%), with two cases showing mutations in APC, ERBB2, and SMARCC1. Common genomic alterations, irrespective of immune status, included gains in copy numbers at 1q, 8q, 17q, and 20q, and losses at 11q, 17p, and 22q. Signature analysis highlighted the predominance of signatures 2 and 1, with “immune cold” samples showing a significant presence of signature 8. Our methylome study on 13 DCIS samples identified 328 hyperdifferentially methylated regions (DMRs) and 521 hypo-DMRs, with “immune cold” cases generally showing lower levels of methylation.
Conclusion
In summary, the molecular characteristics of young-onset DCIS share similarities with invasive breast cancer (IBC), potentially indicating a poor prognosis. Understanding these characteristics, especially the immune microenvironment of DCIS, could be pivotal in identifying new therapeutic targets and preventive strategies for breast cancer.





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study can be available from the corresponding author on reasonable request, following ethics committee approval and a data transfer agreement. Please contact the corresponding author, S.W. (email address: wangshs@sysucc.org.cn) to request access to the data.
References
Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N et al (2022) Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 135(5):584–590. https://doi.org/10.1097/cm9.0000000000002108
Ryser MD, Hendrix LH, Worni M, Liu Y, Hyslop T, Hwang ES (2019) Incidence of ductal carcinoma in situ in the United States, 2000–2014. Cancer Epidemiol Biomarkers Prev 28(8):1316–1323. https://doi.org/10.1158/1055-9965.Epi-18-1262
Bluekens AM, Holland R, Karssemeijer N, Broeders MJ, den Heeten GJ (2012) Comparison of digital screening mammography and screen-film mammography in the early detection of clinically relevant cancers: a multicenter study. Radiology 265(3):707–714. https://doi.org/10.1148/radiol.12111461
Sanders ME, Schuyler PA, Dupont WD, Page DL (2005) The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer 103(12):2481–2484. https://doi.org/10.1002/cncr.21069
Page DL, Dupont WD, Rogers LW, Landenberger M (1982) Intraductal carcinoma of the breast: follow-up after biopsy only. Cancer 49(4):751–758
Maxwell AJ, Clements K, Hilton B, Dodwell DJ, Evans A, Kearins O, Pinder SE, Thomas J, Wallis MG, Thompson AM (2018) Risk factors for the development of invasive cancer in unresected ductal carcinoma in situ. Eur J Surg Oncol 44(4):429–435. https://doi.org/10.1016/j.ejso.2017.12.007
Vicini FA, Shaitelman S, Wilkinson JB, Shah C, Ye H, Kestin LL, Goldstein NS, Chen PY, Martinez AA (2013) Long-term impact of young age at diagnosis on treatment outcome and patterns of failure in patients with ductal carcinoma in situ treated with breast-conserving therapy. Breast J 19(4):365–373. https://doi.org/10.1111/tbj.12127
Mamtani A, Nakhlis F, Downs-Canner S, Zabor EC, Morrow M, King TA, Van Zee KJ (2019) Impact of age on locoregional and distant recurrence after mastectomy for ductal carcinoma in situ with or without microinvasion. Ann Surg Oncol 26(13):4264–4271. https://doi.org/10.1245/s10434-019-07693-1
Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P (2015) Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol 1(7):888–896. https://doi.org/10.1001/jamaoncol.2015.2510
Nagasawa S, Kuze Y, Maeda I, Kojima Y, Motoyoshi A, Onishi T, Iwatani T, Yokoe T, Koike J, Chosokabe M et al (2021) Genomic profiling reveals heterogeneous populations of ductal carcinoma in situ of the breast. Commun Biol 4(1):438. https://doi.org/10.1038/s42003-021-01959-9
Pang JB, Savas P, Fellowes AP, Mir Arnau G, Kader T, Vedururu R, Hewitt C, Takano EA, Byrne DJ, Choong DY et al (2017) Breast ductal carcinoma in situ carry mutational driver events representative of invasive breast cancer. Mod Pathol 30(7):952–963. https://doi.org/10.1038/modpathol.2017.21
Abba MC, Gong T, Lu Y, Lee J, Zhong Y, Lacunza E, Butti M, Takata Y, Gaddis S, Shen J et al (2015) A molecular portrait of high-grade ductal carcinoma in situ. Cancer Res 75(18):3980–3990. https://doi.org/10.1158/0008-5472.Can-15-0506
Lin CY, Vennam S, Purington N, Lin E, Varma S, Han S, Desa M, Seto T, Wang NJ, Stehr H et al (2019) Genomic landscape of ductal carcinoma in situ and association with progression. Breast Cancer Res Treat 178(2):307–316. https://doi.org/10.1007/s10549-019-05401-x
Vincent-Salomon A, Lucchesi C, Gruel N, Raynal V, Pierron G, Goudefroye R, Reyal F, Radvanyi F, Salmon R, Thiery JP et al (2008) Integrated genomic and transcriptomic analysis of ductal carcinoma in situ of the breast. Clin Cancer Res 14(7):1956–1965. https://doi.org/10.1158/1078-0432.Ccr-07-1465
Fleischer T, Frigessi A, Johnson KC, Edvardsen H, Touleimat N, Klajic J, Riis ML, Haakensen VD, Wärnberg F, Naume B et al (2014) Genome-wide DNA methylation profiles in progression to in situ and invasive carcinoma of the breast with impact on gene transcription and prognosis. Genome Biol 15(8):435. https://doi.org/10.1186/preaccept-2333349012841587
Johnson KC, Koestler DC, Fleischer T, Chen P, Jenson EG, Marotti JD, Onega T, Kristensen VN, Christensen BC (2015) DNA methylation in ductal carcinoma in situ related with future development of invasive breast cancer. Clin Epigenetics 7(1):75. https://doi.org/10.1186/s13148-015-0094-0
Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH (2016) Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol 34:539–573. https://doi.org/10.1146/annurev-immunol-032414-112049
Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børresen-Dale AL et al (2013) Signatures of mutational processes in human cancer. Nature 500(7463):415–421. https://doi.org/10.1038/nature12477
Rubin AF, Green P (2009) Mutation patterns in cancer genomes. Proc Natl Acad Sci U S A 106(51):21766–21770. https://doi.org/10.1073/pnas.0912499106
Stephens PJ, Tarpey PS, Davies H, Van Loo P, Greenman C, Wedge DC, Nik-Zainal S, Martin S, Varela I, Bignell GR et al (2012) The landscape of cancer genes and mutational processes in breast cancer. Nature 486(7403):400–404. https://doi.org/10.1038/nature11017
Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61-70. doi:https://doi.org/10.1038/nature11412
Bertucci F, Ng CKY, Patsouris A, Droin N, Piscuoglio S, Carbuccia N, Soria JC, Dien AT, Adnani Y, Kamal M et al (2019) Genomic characterization of metastatic breast cancers. Nature 569(7757):560–564. https://doi.org/10.1038/s41586-019-1056-z
Law EK, Sieuwerts AM, LaPara K, Leonard B, Starrett GJ, Molan AM, Temiz NA, Vogel RI, Meijer-van Gelder ME, Sweep FC et al (2016) The DNA cytosine deaminase APOBEC3B promotes tamoxifen resistance in ER-positive breast cancer. Sci Adv 2(10):e1601737. https://doi.org/10.1126/sciadv.1601737
Sannigrahi MK, Srinivas S, Rakshit S (2018) The prospects of cadherin-23 as a mediator of homophilic cell–cell adhesion. Adv Exp Med Biol 1112:99–105. https://doi.org/10.1007/978-981-13-3065-0_8
Swann JB, Smyth MJ (2007) Immune surveillance of tumors. J Clin Invest 117(5):1137–1146. https://doi.org/10.1172/jci31405
Kim M, Chung YR, Kim HJ, Woo JW, Ahn S, Park SY (2020) Immune microenvironment in ductal carcinoma in situ: a comparison with invasive carcinoma of the breast. Breast Cancer Res 22(1):32. https://doi.org/10.1186/s13058-020-01267-w
Toss MS, Abidi A, Lesche D, Joseph C, Mahale S, Saunders H, Kader T, Miligy IM, Green AR, Gorringe KL et al (2020) The prognostic significance of immune microenvironment in breast ductal carcinoma in situ. Br J Cancer 122(10):1496–1506. https://doi.org/10.1038/s41416-020-0797-7
Toss MS, Miligy I, Al-Kawaz A, Alsleem M, Khout H, Rida PC, Aneja R, Green AR, Ellis IO, Rakha EA (2018) Prognostic significance of tumor-infiltrating lymphocytes in ductal carcinoma in situ of the breast. Mod Pathol 31(8):1226–1236. https://doi.org/10.1038/s41379-018-0040-8
Thompson E, Taube JM, Elwood H, Sharma R, Meeker A, Warzecha HN, Argani P, Cimino-Mathews A, Emens LA (2016) The immune microenvironment of breast ductal carcinoma in situ. Mod Pathol 29(3):249–258. https://doi.org/10.1038/modpathol.2015.158
McCleskey BC, Penedo TL, Zhang K, Hameed O, Siegal GP, Wei S (2015) GATA3 expression in advanced breast cancer: prognostic value and organ-specific relapse. Am J Clin Pathol 144(5):756–763. https://doi.org/10.1309/ajcp5mmr1fjvvtpk
Van Coillie S, Wiernicki B, Xu J (2020) Molecular and cellular functions of CTLA-4. Adv Exp Med Biol 1248:7–32. https://doi.org/10.1007/978-981-15-3266-5_2
Wang H, Xie X, Zhu J, Qi S, Xie J (2021) Comprehensive analysis identifies IFI16 as a novel signature associated with overall survival and immune infiltration of skin cutaneous melanoma. Cancer Cell Int 21(1):694. https://doi.org/10.1186/s12935-021-02409-6
Ka NL, Lim GY, Kim SS, Hwang S, Han J, Lee YH, Lee MO (2022) Type I IFN stimulates IFI16-mediated aromatase expression in adipocytes that promotes E(2)-dependent growth of ER-positive breast cancer. Cell Mol Life Sci 79(6):306. https://doi.org/10.1007/s00018-022-04333-y
Khan MI, Nur SM, Abdulaal WH (2022) A study on DNA methylation modifying natural compounds identified EGCG for induction of IFI16 gene expression related to the innate immune response in cancer cells. Oncol Lett 24(1):218. https://doi.org/10.3892/ol.2022.13339
Karasawa T, Kawashima A, Usui F, Kimura H, Shirasuna K, Inoue Y, Komada T, Kobayashi M, Mizushina Y, Sagara J et al (2015) Oligomerized CARD16 promotes caspase-1 assembly and IL-1β processing. FEBS Open Bio 5:348–356. https://doi.org/10.1016/j.fob.2015.04.011
Zardavas D, Te Marvelde L, Milne RL, Fumagalli D, Fountzilas G, Kotoula V, Razis E, Papaxoinis G, Joensuu H, Moynahan ME et al (2018) Tumor PIK3CA genotype and prognosis in early-stage breast cancer: a pooled analysis of individual patient data. J Clin Oncol 36(10):981–990. https://doi.org/10.1200/jco.2017.74.8301
Acknowledgements
This research was supported by the Guangdong Provincial Key Laboratory of Human Disease Genomics (2020B1212070028). This work was supported by the China National GeneBank (CNGB).
Funding
This work was supported by the National Natural Science Foundation of China (82372590, 82303007), Natural Science Foundation of Guangdong Province (2023A1515030092, 2020A1515010105), Guangdong Basic and Applied Basic Research Foundation (2022A1515111202), Fostering Program for NSFC Young Applicants (Tulip Talent Training Program) of Sun Yat-sen University Cancer Center (2023yfd10).
Author information
Authors and Affiliations
Contributions
Concept and design: WZ, SW, KS. Acquisition, analysis, or interpretation of data: RH, BC, DC. Drafting of the manuscript: DC. Critical revision of the manuscript for important intellectual content: RH. Statistical analysis: BC. Administrative, technical, or material support: WW, TL, DL. Supervision: WZ, SW, KS.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The study was approved by the ethical committee of Sun Yat-sen University Cancer Center and waived the requirement for informed consent due to the retrospective nature of this study.
Consent to participant
The study waived the requirement for informed consent due to the retrospective nature of this study.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hong, R., Cao, B., Chen, D. et al. Multi-omics portrait of ductal carcinoma in situ in young women. Breast Cancer Res Treat (2024). https://doi.org/10.1007/s10549-024-07254-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10549-024-07254-5