Abstract
Purpose
HER2 overexpression has a central role in breast cancer carcinogenesis and is associated with poor prognosis if untreated. Lately, identification of HER2-low breast cancer has been proposed to select patients for novel HER2-directed chemotherapy and includes cancers with immunohistochemistry 1 + or 2 + with negative FISH, encompassing approximately 55–60% of all breast carcinomas. In early-stage breast cancer, the prognostic significance of HER2 low-disease is less well understood, with a particular paucity of data evaluating the prevalence and implications of HER2-low status in invasive lobular carcinoma (ILC).
Methods
We evaluated 666 stage I-III ILC tumors from a prospectively maintained institutional database, comparing clinicopathologic features and disease-free survival (DFS) using a multivariable Cox proportional hazards model.
Results
HER2-low status was common in this cohort of patients with ILC, but most clinicopathologic features did not differ between HER2-low and HER2-negative cases. However, when adjusting for tumor size, number of positive nodes, ER/PR status, and local therapy received, patients with HER2-low status had worse disease-free survival (DFS) than those with HER2-negative tumors (hazard ratio 2.0, 95% confidence interval 1.0–4.1, p = 0.05).
Conclusion
This difference in DFS supports the notion that HER2-low and HER2-negative early stage ILC may differ clinically, despite similar clinicopathologic features. Further investigation into the potential benefit of HER2 targeted therapy in HER2-low early-stage breast cancer, and specifically lobular cancer, is warranted to ensure optimal outcomes in this distinct tumor subtype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human epidermal growth factor receptor 2 (HER2) is a family of transmembrane receptor tyrosine kinases encoded by the ERBB2 gene, which has been an important area of research and treatment target in breast cancer [1]. Amplification of the ERBB2 gene results in HER2 overexpression, which has a central role in promoting carcinogenesis in breast cancer and is associated with poor prognosis in untreated patients [2]. However, since the discovery of the monoclonal HER2 antibody trastuzumab, HER2 overexpression has predicted response to anti-HER2 treatment [3, 4]. Additionally, novel HER2-targeting antibody–drug conjugates (ADCs) have provided another way to potentially target HER2 in tumors that show low expression of HER2 [5]. A recent Phase 3 randomized trial demonstrated improved progression-free and overall survival for those with HER2-low disease treated with the ADC trastuzumab deruxtecan (NCT03734029/DESTINY-Breast04) in the metastatic setting [6].
Hence, there is new interest to define cancers with low levels of HER2 expression for this therapeutic approach, such as nonamplified (FISH-negative) tumors with immunohistochemistry (IHC) scores of 1 + or 2 + . By this definition, approximately 55–60% of breast carcinomas are HER2-low, of which 80% are hormone receptor positive and 15–20% are hormone receptor negative [1]. In clinical practice, these tumors are traditionally considered HER2-negative, since agents targeting biological dependence on the HER2 pathway, such as trastuzumab, have not been shown to offer clinical benefit [7]. In early-stage breast cancer, the prognostic significance of HER2 low-disease is less well understood, with a recent retrospective analysis showing no difference in outcomes by HER2-low status [5].
The prevalence and implications of HER2-low status in invasive lobular carcinoma (ILC) is currently unknown. ILC is the second most common type of breast cancer after invasive ductal carcinoma (IDC), representing 10–15% of all breast cancer. The majority of ILCs are hormone receptor (HR) positive and HER2-negative. Previous studies of HER2-low status in breast cancer consistently find a lower prevalence of HER2-low status in ILC compared to IDC [8,9,10]. To our knowledge, no studies have reported the prognostic significance of HER2-low status in early stage ILC.
In the current study, we aimed to describe the prevalence of HER2-low status in a mono-institutional cohort of early-stage ILC, with emphasis on clinicopathologic features and clinical outcome.
Methods
With institutional review board approval, we retrieved clinicopathologic data from a prospectively maintained institutional database containing treatment and outcomes data for ILC patients undergoing surgery at the University of California, San Francisco between January 1996 and September 2019.
Population
We included patients with tumors that had lobular or mixed lobular/ductal histology, and were stage I-III. HR positivity was defined as having ≥ 1% either estrogen receptor (ER) or progesterone receptor (PR) expression by immunohistochemistry. Both IHC and FISH testing for HER2 status were performed as part of standard clinical care on all cases. Tumors were classified as HER2-negative (IHC = 0), HER2-low (IHC = 1 + or 2 + with negative FISH [ratio of HER2/CEP17 < 2]), or HER2-positive (IHC = 3 + or FISH ratio ≥ 2).
Clinicopathological parameters
The following baseline clinicopathological parameters were evaluated by HER2 status: age at diagnosis, menopausal status, body mass index (BMI), tumor stage, tumor histology, tumor grade, ER/PR status, molecular risk score (21-gene Recurrence Score or 70 gene signature when available), type of breast surgery, timing of chemotherapy administration (neoadjuvant or adjuvant). High 21-gene recurrence score (RS) risk was defined as including patients with a RS of 26 or greater, the intermediate-risk group as including patients with an RS between 11 and 25, and the low-risk group as including patients with an RS of 10 or less [12]. The 70 gene signature RS was categorized as high risk and low risk [13, 14]. Data were analyzed and reported using essential elements of REMARK criteria [15].
Statistical analysis
The primary study end point was disease-free survival (DFS). DFS was defined as time from the date of primary surgery to the date of disease recurrence or death; patients alive without disease recurrence were censored at the date of last follow-up. Disease free survival was analyzed using the log rank test and Kaplan Meier method, and multivariate Cox proportional hazards models to estimate hazard ratios with 95% confidence intervals (CI) among HER2-negative and HER2-low cases with at least 6 months of follow up time and right-censored at 10 years. Data were analyzed in Stata 16.1 using chi-squared tests and t-tests. Data were analyzed between February 2022 and April 2022.
Results
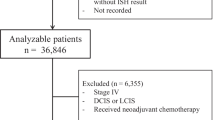
Six-hundred and sixty-six ILCs with available HER2 status were included in the analysis (Table 1). The mean age at diagnosis was 59.8 years (range 21–91), and the majority of patients were post-menopausal (69.3%). There were 408 (63.1%) patients with stage I disease, 160 (24.7%) with stage II, and 79 (12.2%) with stage III disease. Most tumors were grade 2 (n = 448, 68.5%), with 168 (25.7%) grade 1 and 38 (5.8%) being grade 3. The mean follow-up time was 6.7 years (standard deviation 5.4).
HER2-low status was common in the study cohort. Overall, 184 (27.6%) tumors were HER2-negative, 434 (65.1%) were HER2-low, and 48 (7.2%) were HER2-positive. HER2 status was associated with hormone receptor status, as HER2-low tumors were more likely to have PR positivity than both HER2-negative and HER2-positive tumors (86.6% compared to 79.9% of HER2-negative and 72.9% of HER2-positive tumors, p = 0.01). This difference remained significant when comparing HER2-low to HER2-negative tumors and excluding HER2-positive tumors (p = 0.034). However, there were no associations between HER2-low status and patient characteristics such as age, menopausal status, or BMI. Similarly, HER2-low status was not associated with tumor features including stage, grade, presence of lympho-vascular invasion, lobular carcinoma in situ, or multifocal disease. Within a subset of 304 HER2-negative or HER2-low tumors with either 70 gene signature results (n = 121) or 21-gene Recurrence Scores (n = 183) available, there was no association between HER2 status and molecular assay result.
HER2 positivity was significantly associated with a higher rate of pleomorphic ILC when compared to HER2-negative and HER2-low cases (33% versus 9.8% and 9.9% respectively, p > 0.001). Additionally, HER2-positive tumors were significantly less likely to be ER positive than both HER2-negative and HER2-low tumors (89.6% versus 97.3% and 96.8% respectively, p = 0.03).
Regarding treatment, we found no differences in use of neoadjuvant or adjuvant chemotherapy by HER2 status when comparing HER2-low to HER2-negative cases. However, patients with HER2-low tumors were more likely to undergo mastectomy versus lumpectomy when compared to HER2-negative and HER2-positive patients (53.7% versus 38.0% and 43.8% respectively, p = 0.001).
In unadjusted evaluation of DFS by the log rank test, there was no difference between HER2-negative and HER2-low cases. However, in a multivariable Cox proportional hazards model adjusting for tumor size, number of positive nodes, ER/PR status, and local therapy received, patients with HER2-low status had worse DFS than those with HER2-negative tumors (HR 2.0, 95% CI 1.0–4.1, p = 0.05) (Table 2). In this model, other factors associated with worse DFS included larger tumor size (HR 1.13, 95% CI 1.04–1.2, p = 0.005), increasing number of positive nodes (HR 1.1, 95% CI 1.1–1.13, p < 0.001), and undergoing lumpectomy without radiation for local therapy (HR 3.4, 95% CI 1.6–7.6, p = 0.002); PR positivity was associated with improved DFS compared to PR negativity (HR 0.4, 95% CI 0.2–0.8, p = 0.008).
Discussion
In this study of 666 women with early-stage ILC, we found that the majority (65.1%) of tumors were HER2-low. Although two prior studies suggested that HER2-low positivity may be less likely in those with ILC [8, 9], the prevalence of HER2-low status in our cohort is similar to what is reported in hormone receptor positive breast cancer, irrespective of histology [1, 8, 16]. The prevalence of HER2-low ILC in our cohort supports a recently published study that compiled data from various datasets and included roughly 500 patients with ILC [17]. By contrast, a single institution retrospective review with fewer patients found a higher prevalence of HER2-low ILC compared to IDC [18].
We found very few differences in clinicopathologic features between HER2-low and HER2-negative disease. Interestingly, we found that that those with HER2-low disease were significantly more likely to have PR positive tumors than those with either HER2-negative or HER2-positive disease. Since PR positivity is usually associated with improved prognosis, the significance of this finding is unclear, especially since we found that HER2-low cases in this cohort had modestly but significantly worse DFS that HER2-negative cases. This difference in PR status may reflect other underlying biologic differences between HER2-low and HER2-negative ILC which needs further investigation. In our cohort, HER2-low ILC patients were more likely to have had a mastectomy versus lumpectomy, while a recent large study of early-stage breast cancer showed no association between type of breast surgery and HER2 status [11]. The underlying cause for increased mastectomies in HER2-low patients in our cohort is unclear, as we did not find an association between tumor size, age, or multifocality and HER2 status.
The current literature provides mixed results regarding the prognostic significance of HER2-low status in early-stage breast cancer show. Two large retrospective studies from Japan and Korea found no difference in overall survival between early-stage HR positive HER2-low and HER2-negative patients [10, 18] On the other hand, a recently published study of early-stage HR positive breast cancer found that while HER2-low status was not associated with recurrence free survival overall, there was a trend towards increased risk of recurrence specifically among those with lobular histology and HER2-low disease [8]. In our analysis, we similarly found an association between HER2-low status and worse outcomes in those with ILC when adjusting for local therapy, hormone receptor status, tumor size, and number of positive nodes. Our findings combined with the previously reported trend suggest that HER2-low status should be explored further in early-stage breast cancer, and ILC specifically. This is particularly relevant given the recent reports of clinical activity using the ADCs in HER2-low metastatic breast cancers, and the possibility that some strategies of targeting HER2 might be effective in early-stage HER2-low disease as well [19, 20].
One limitation of this study and others is that HER2 IHC interpretation is observer dependent. In our institution, HER2 IHC is reviewed by subspecialized breast pathologists as part of routine clinical care, and cases undergo reflex FISH testing, irrespective of IHC score. Currently, there remains no assay to reliably identify HER2-low breast cancers. Another limitation is that this data is a retrospective study from a single cancer center, and thus may not be representative of a larger population. Additionally, retrospective analyses are subject to treatment selection bias.
However, our findings support the notion that HER2-low and HER2-negative disease may represent two different clinical entities within ILC. It should be noted that there is an important distinction between molecular classification based on biological drivers (discriminants of the disease itself) and the drug targets designed into novel ADCs. Given the recent finding of therapeutic benefit of ADCs in HER2-low breast cancer in the metastatic setting, identifying potential tumor types that might derive benefit of such approaches in the early-stage setting is of clinical interest. Further investigation of the potential benefit of HER2 targeted therapy in ILC, which predominately lacks HER2-amplified disease, is warranted to ensure optimal outcomes in this understudied tumor subtype currently treated similarly to the more common ductal cancers.
Conclusions
Overall, our findings illustrate a high prevalence of HER2-low disease in early stage ILC. Further studies are required to better understand HER2-low breast cancer in general, and in particular in ILC, the second most common type of breast cancer.
Data availability
The data supporting all tables in this published article are not publicly available to protect patient privacy, but can be accessed from the corresponding author on request. Data will be made available to authorized researchers who have obtained Institutional Review Board (IRB) approval from their own institution and from the University of California, San Francisco IRB.
Abbreviations
- HER2:
-
Human epidermal growth factor receptor 2
- ADC:
-
Antibody-drug conjugates
- FISH:
-
Fluorescent in situ hybridization
- ILC:
-
Invasive lobular carcinoma
- IDC:
-
Invasive ductal carcinoma
- HR:
-
Hormone receptor
- IHC:
-
Immunohistochemistry
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- BMI:
-
Body mass index
- RS:
-
Recurrence score
- DFS:
-
Disease free survival
References
Marchiò C, Annaratone L, Marques A, Casorzo L, Berrino E, Sapino A (2021) Evolving concepts in HER2 evaluation in breast cancer: Heterogeneity, HER2-low carcinomas and beyond. Semin Cancer Biol 72:123–135. https://doi.org/10.1016/j.semcancer.2020.02.016
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/ neu oncogene. Science 235(4785):177–182. https://doi.org/10.1126/science.3798106
Hayes DF (2019) HER2 and Breast Cancer — A Phenomenal Success Story. Phimister EG, editor. N Engl J Med. 381(13):1284–6. https://doi.org/10.1056/NEJMcibr1909386
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS et al (2018) Human epidermal growth factor receptor 2 testing in breast cancer: american society of clinical oncology/college of american pathologists clinical practice guideline focused update. Arch Pathol Lab Med 142(11):1364–1382. https://doi.org/10.1200/JCO.2018.77.8738
Tarantino P, Hamilton E, Tolaney SM, Cortes J, Morganti S, Ferraro E et al (2020) HER2-low breast cancer: pathological and clinical landscape. JCO 38(17):1951–1962. https://doi.org/10.1200/JCO.19.02488
Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E et al (2022) Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 387(1):9–20. https://doi.org/10.1056/NEJMoa2203690
Fehrenbacher L, Cecchini RS, Geyer CE Jr, Rastogi P, Costantino JP, Atkins JN et al (2020) NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2+. JCO 38(5):444–453. https://doi.org/10.1200/JCO.19.01455
Douganiotis G, Kontovinis L, Markopoulou E, Ainali A, Zarampoukas T, Natsiopoulos I, et al. (2022) Prognostic Significance of Low HER2 Expression in Patients With Early Hormone Receptor Positive Breast Cancer. Cancer Diag and Prognosis 2(3):316–23. https://doi.org/10.21873/cdp.10111
Mutai R, Barkan T, Moore A, Sarfaty M, Shochat T, Yerushalmi R et al (2021) Prognostic impact of HER2-low expression in hormone receptor positive early breast cancer. The Breast 60:62–69. https://doi.org/10.1016/j.breast.2021.08.016
Won HS, Ahn J, Kim Y, Kim JS, Song JY, Kim HK et al (2022) Clinical significance of HER2-low expression in early breast cancer: a nationwide study from the Korean Breast Cancer Society. Breast Cancer Res 24(1):22. https://doi.org/10.1186/s13058-022-01519-x
Tarantino P, Jin Q, Tayob N, Jeselsohn RM, Schnitt SJ, Vincuilla J et al (2022) Prognostic and biologic significance of ERBB2-low expression in early-stage breast cancer. JAMA Oncol 8(8):1177–1183. https://doi.org/10.1001/jamaoncol.2022.2286
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF et al (2018) Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 379(2):111–121. https://doi.org/10.1056/NEJMoa1804710
Cardoso F, van’t Veer L J, Bogaerts J, Slaets L, Viale G, Delaloge S, et al. (2016) 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 375(8):717–29. https://doi.org/10.1056/NEJMoa1602253
Buyse M, Loi S, van’t Veer L, Viale G, Delorenzi M, Glas AM, et al. (2006) Validation and Clinical Utility of a 70-Gene Prognostic Signature for Women With Node-Negative Breast Cancer. JNCI: Journal of the National Cancer Institute 98(17):1183–92. https://doi.org/10.1093/jnci/djj329
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2006) Reporting recommendations for tumor marker prognostic studies (REMARK). Breast Cancer Res Treat 100(2):229–235. https://doi.org/10.1007/s10549-006-9242-8
Zhang H, Katerji H, Turner BM, Hicks DG (2022) HER2-low breast cancers. Am J Clin Pathol 157(3):328–336. https://doi.org/10.1038/s41379-022-01019-5
Schettini F, Chic N, Brasó-Maristany F, Paré L, Pascual T, Conte B, et al. (2021) Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. npj Breast Cancer 7(1):1. https://doi.org/10.1038/s41523-020-00208-2
Horisawa N, Adachi Y, Takatsuka D, Nozawa K, Endo Y, Ozaki Y et al (2022) The frequency of low HER2 expression in breast cancer and a comparison of prognosis between patients with HER2-low and HER2-negative breast cancer by HR status. Breast Cancer 2:234–241. https://doi.org/10.1007/s12282-021-01303-3
Banerji U, van Herpen CML, Saura C, Thistlethwaite F, Lord S, Moreno V et al (2019) Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol 8:1124–1135. https://doi.org/10.1016/S1470-2045(19)30328-6
Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J et al (2020) Antitumor activity and safety of Trastuzumab Deruxtecan in patients with HER2-low–expressing advanced breast cancer: results from a phase Ib study. JCO 38(17):1887–1896. https://doi.org/10.1200/JCO.19.02318
Funding
RAM was supported by the National Cancer Institute Award K08CA256047. HTR was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number TL1 TR 001871. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Contributions
RAM contributed to the study conception and design. Material preparation, data collection and analysis were performed by RAM, HTR, EL, AP, JMV, and MK. The first draft of the manuscript was written by HTR and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Symmans’ holds licensed invention patents (not related to this research) founder equity in Delphi Diagnostics, owns shares in IONIS Pharmaceuticals and Eiger Biopharmaceuticals, research funding from Pfizer, consultant to Astra Zeneca. A. Jo Chien receives research funding from Merck, Puma, Amgen, and Seattle Genetics. All other authors have no financial or non-financial conflict of interests to disclose.
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the IRB of the University of California, San Francisco (17–23,655, January 16, 2020).
Consent to participate
Informed consent requirement was waived by the IRB, as no subjects were contacted for this study.
Consent to publication
Consent for publication was waived by the IRB, as no subjects were contacted for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rothschild, H.T., Clelland, E., Patterson, A. et al. HER-2 low status in early-stage invasive lobular carcinoma of the breast: associated factors and outcomes in an institutional series. Breast Cancer Res Treat 199, 349–354 (2023). https://doi.org/10.1007/s10549-023-06927-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-06927-x