Abstract
Purpose
In nonmetastatic hormone receptor-positive and Her2-negative breast cancer, preoperative endocrine therapies can yield outcomes similar with chemotherapy. We evaluated the tolerability and preliminary antitumor activity of preoperative letrozole, everolimus, and carotuximab, a monoclonal antibody targeting endoglin, in nonmetastatic breast cancer.
Methods
Eligible patients had newly diagnosed, stage 2 or 3, hormone receptor-positive and Her2/neu-negative breast cancer. Patients received escalating doses of everolimus; the dose of letrozole and carotuximab were fixed at 2.5 mg PO daily and 15 mg/kg intravenously every 2 weeks, respectively. The primary objective was to determine the safety and tolerability of the combination. Secondary objectives included pharmacokinetic and pharmacodynamic studies and assessments of antitumor activity.
Results
Fifteen patients enrolled. The recommended phase 2 dose of everolimus in combination with letrozole and carotuximab was 10 mg PO daily. The most frequent adverse events were headache (67%), fatigue (47%), facial flushing and swelling (47%), gingival hemorrhage (40%), epistaxis (33%), nausea and vomiting (27%). Headache constituted a dose-limiting toxicity. At least two signs of mucocutaneous telangiectasia developed in 92% of patients. Carotuximab accumulated in the extravascular space and accelerated the biodistribution and clearance of everolimus. All patients had residual disease. Gene expression analyses were consistent with downregulation of genes involved in proliferation and DNA repair. Among 6 patients with luminal B breast cancer, 5 converted to luminal A after one cycle of therapy.
Conclusion
Letrozole, everolimus, and carotuximab were tolerated in combination at their single-agent doses. Pharmacokinetic studies revealed an interaction between everolimus and carotuximab.
Trial registration
This trial is registered with ClinicalTrials.gov (Identifier: NCT02520063), first posted on August 11, 2015, and is active, not recruiting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In non-metastatic breast cancer, the achievement of a pathologic complete remission (pCR) with preoperative therapy has been consistently associated with better long-term outcomes[1, 2]. In hormone receptor-positive and Her2-negative (HR+/Her2−) breast cancer, preoperative chemotherapy and endocrine therapy, have been shown to yield similar benefits, albeit in unselected patients, with endocrine therapy being associated with significantly fewer adverse events[3]. Despite the high rates of clinical and radiographic responses with preoperative endocrine therapy[4,5,6,7,8], the rates of downstaging and more so pCR are still low [7].
Exploiting therapeutically the interplay between angiogenesis and estrogen receptor (ER), we have shown in 2 separate clinical trials that the addition of bevacizumab, a monoclonal antibody targeting the vascular endothelial growth factor (VEGF), to letrozole, leads to pCR rates (11–12%) comparable to preoperative chemotherapy [3, 9, 10]. The addition of mammalian Target of Rapamycin (mTOR) inhibitor to hormonal therapy reverses endocrine resistance and enhances the efficacy of hormonal agents [11,12,13,14]. mTOR inhibition abrogates VEGF-mediated signaling which, in turn, leads to compensatory overexpression of endoglin (CD105) in the endothelial cells [15, 16]. Upon VEGF inhibition, CD105(+) vessels in the periphery and core of tumors persist or regenerate [16]. Thus, the addition of an anti-CD105 agent may increase the antitumor efficacy seen in a randomized trial of preoperative letrozole and everolimus [12].
Endoglin (CD105) is a transmembrane accessory receptor for transforming growth factor-beta (TGF-β) that is predominantly expressed on proliferating endothelial cells and angiogenic blood vessels [17] as opposed to the normal quiescent endothelium which has a very low turnover [18, 19]. It diverts TGF-β downstream signaling toward proliferation and migration and promotes transcription of pro-angiogenic genes including endoglin itself [20,21,22]. Carotuximab (TRC105) is a chimeric immunoglobulin G1 monoclonal antibody that binds to endoglin, inhibits TGF-β mediated pro-angiogenic signaling, and induces potent antibody-dependent cell-mediated cytotoxicity[23]. In preclinical murine xenograft models of HR(+) breast cancer, anti-endoglin monoclonal antibodies selectively inhibited tumor angiogenesis and delayed the growth of established tumors, while sparing normal vasculature [24].
The purpose of the study was to determine the safety of combining everolimus and carotuximab with letrozole (with goserelin if premenopausal) in women with newly diagnosed stage 2 and 3 HR+/Her2− breast cancer. Secondary objectives included determination of the efficacy, pharmacokinetic and pharmacodynamic parameters of the triple combination. The study was terminated early because the clinical development of carotuximab was halted.
Methods
Patient eligibility
Eligible patients had newly diagnosed, potentially resectable, pathologically confirmed invasive stage 2 and 3 breast cancer (clinical T2, T3, T4a-c, N0-2, and M0), ER- and/or progesterone receptor (PR)-positive, Her2/neu-negative [25, 26]. At least 10% of tumor cell nuclei had to be immunoreactive for ER and/or PR. Breast magnetic resonance imaging (MRI) were performed at baseline, week 12 and 24. Patients with multicentric, multifocal, or bilateral breast cancer were allowed, so long as all separate tumors met eligibility criteria.
The protocol was amended once to allow premenopausal women whose ovarian function was to be suppressed with goserelin to participate in the study. The protocol was reviewed and approved by the participating institution, and the study followed the Declaration of Helsinki and Good Clinical Practice guidelines. All patients gave informed consent. This study is registered on the clinical trial website of the US National Cancer Institute (https://clinicaltrials.gov/ct2/show/NCT02520063).
Study design and treatments
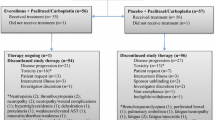
This was a single-institution study with a dose escalation (phase 1) and expansion (phase 2) part (Fig. 1A). During the first part, the Maximum Tolerated Dose (MTD) and the Recommended Phase 2 Dose (RP2D) of the combination was determined. The dose of everolimus was escalated from 5 (cohort 1) to 10 mg PO daily (cohort 2). Everolimus was to be de-escalated to 5 mg PO daily (cohort 1) if 10 mg PO daily was not well tolerated. The dose of carotuximab was 15 mg/kg IV q 2 weeks (cohorts 1 and 2). Dose calculations were capped to a weight of 85 kg. The dose of carotuximab was to be de-escalated to 10 mg/kg IV q 2 weeks (cohort −1) if 15 mg/kg IV q 2 weeks in combination with everolimus 5 mg PO daily were not well tolerated. The dose of letrozole was fixed (2.5 mg PO daily). Premenopausal women received goserelin 3.6 mg subcutaneously q 4 weeks. Ovarian function suppression was monitored with estradiol levels on cycle 2 day 1 and q 2 cycles thereafter.
Study Schema and Pharmacokinetics. A The study had 2 parts (phase 1 and 2). Escalating doses of everolimus and carotuximab are shown in the x and y axis, respectively. The dose of letrozole and goserelin in premenopausal women was fixed in all cohorts. The thick black arrow indicates direction of dose escalation while the thin grey arrow indicates direction of de-escalation. The recommended phase 2 dose for everolimus and carotuximab was 10 mg PO daily and 15 mg/kg IV q 2 weeks, respectively. Five patients enrolled in the phase 2 part before the trial closed. (SAE, serious adverse event). B Spaghetti plot of the measured on cycle 2 day 1 and estimated steady-state concentrations of letrozole. Note the measured concentrations of letrozole being consistently higher than estimated indicative of accumulation and non-linear pharmacokinetic properties. C Spaghetti plot of the estimated steady-state concentrations of everolimus (color coded for dose). Note the lower than previously reported Cmax (solid arrow) and the rapid postabsorption distribution (empty arrow) of the 10 mg everolimus. D Simulated carotuximab concentration–time curves over the course of protocol therapy. Intravascular (continuous lines) and extravascular (dotted lines) concentrations color coded for 2 simulations: intravascular volume of 39 mL/kg, extravascular volume of distribution (137–39=) 98 mL/kg, intercompartmental clearance 27 mL/h/kg [59], clearance 0.321 (simulation 1) and 0.464 (simulation 2) [23] mL/kg/h. Note the rising concentrations of carotuximab in the extravascular space with successive administrations of carotuximab
Letrozole was continued until the day prior to surgery. For everolimus and carotuximab, an interval of 4 weeks between last dose and surgery was required. The first dose of carotuximab was split into two infusions, each administered over 4 h, on days 1 (3 mg/kg) and 4 (12 mg/kg or 7 mg/kg if dose de-escalation occurred). If well tolerated, all subsequent infusions were administered as a whole every 2 weeks; infusion time was step-wise reduced to a minimum of 90 min. Premedications for each infusion included acetaminophen 650 mg PO, methylprednisolone 100 mg IV, famotidine 20 mg and cetirizine 10 mg PO or IV.
Delays for up to 3, 7, 7, and 14 days for goserelin, letrozole, everolimus, and carotuximab, respectively, were allowed. If carotuximab were delayed for > 3 days, the first dose of carotuximab upon resumption had to be split into two doses: 3 mg/kg on day 1 and the remainder on day 4. Dose reductions down to 2.5 mg PO daily and 10 mg/kg q 2 weeks were allowed for everolimus and carotuximab, respectively.
Assessments for safety and efficacy
Toxicity was assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03 (https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf). Patients underwent evaluation of response by physical examination and breast ultrasound (at the beginning of every cycle), and breast MRI. Patients with objective response or stable disease according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST [27]) continued the investigational therapy for a maximum of 24 weeks.
Definition of dose-limiting toxicity, pharmacokinetic analyses, nanostring analyses, immunohistochemistry, statistical considerations
See Supplementary Material.
Results
Patient characteristics and disposition
Fifteen patients enrolled and received at least one cycle of the investigational therapy (Table 1). Two patients had bilateral and one multifocal breast cancer. The age range of the patients was broad with near-equal representation of pre- and postmenopausal women. All patients had ECOG performance status 0. The average primary tumor size on MRI was 5.01 cm.
Maximum tolerated dose and dose-limiting toxicities
Ten patients enrolled in the phase 1 part (Fig. 1A). Among the first 3 patients enrolled in cohort 1 (everolimus 5 mg PO daily + carotuximab 15 mg/kg q 2 weeks), one patient discontinued permanently protocol therapy due to intercurrent illness unrelated to protocol therapy (MediPort infection) and one patient had a serious adverse event (“first-dose” headache [see below]). She resumed treatment with 1 week delay and completed protocol therapy without dose reductions or further interruptions. Cohort 1 was expanded to a total of 6 patients; no serious adverse events were noted and the trial proceeded to cohort 2 (everolimus 10 mg PO daily + carotuximab 15 mg/kg q 2 weeks). Four patients enrolled in cohort 2 to account for one patient who was noncompliant and taken off study. No serious adverse events were seen in cohort 2. The RP2D was declared at letrozole 2.5 mg and everolimus 10 mg PO daily + carotuximab 15 mg/kg q 2 weeks ± goserelin 3.6 mg SC q 4 weeks; the MTD was not reached.
Safety and tolerability
All patients experienced adverse events (Table 2). The nature and frequency of adverse events were similar in the two phase I cohorts. The majority of adverse events were mild to moderate in severity (grade 1–2). The profile of adverse events of the combination was dominated by the ones associated with carotuximab [23, 28,29,30,31,32]. Adverse events typically associated with aromatase inhibitors (hot flashes, joint stiffness) and metabolic adverse events typically associated with everolimus (dyslipidemias) arose after cycle 1 and were comparatively less frequent.
At least two signs of mucocutaneous telangiectasia (epistaxis, gingival bleeding, and telangiectasia), that typify hereditary hemorrhagic telangiectasia (HHT) were seen in 40% (6/15) of patients during cycle 1 and 92% (11/12) of patients who continued on the investigational therapy beyond cycle 1. HHT1 or Osler–Rendu–Weber syndrome 1 is a genetic disorder characterized by a deficiency in endoglin. Headache was the most frequent adverse event during cycle 1 and constituted the only dose-limiting toxicity (DLT). As previously reported [32], headache was not associated with hypertension or focal neurologic signs. In the patient in whom imaging was performed, no radiographic abnormalities were seen. “First-dose” headache has been mitigated by splitting the first dose of carotuximab in 2 separate infusions [32], a strategy that was adopted in our trial. Although everolimus has also been associated with headache (11% when used in combination with letrozole [12]), headache was temporally associated with the infusions of carotuximab.
Although the adverse events were mostly grade 1 or 2, they all tended to persist, accumulate, and worsen with subsequent cycles of therapy. These adverse events were most notably fatigue which all patients beyond cycle 1 experienced; normocytic normochromic hypoproliferative anemia suspected to result from carotuximab’s effect on the proerythroblasts [23, 33]; epistaxis which would be triggered by progressively milder stimuli or even occur spontaneously. Although gingival hemorrhage is a distinctive, “on-target” adverse event associated with carotuximab, the higher frequency with which gingival hemorrhage was seen in this trial (92% vs. 31–50% [28, 29, 32]) alongside with gingival tenderness and mucositis may reflect a synergistic effect between everolimus and carotuximab. Similarly, a synergistic effect between carotuximab and everolimus may underpin the universal occurrence of fatigue (reported with both everolimus and carotuximab), and the high frequency of skin rash (typically associated with everolimus, reported in 20% of patients [12] vs. 75% in this study).
Toxicities associated with VEGF inhibition (hypertension, proteinuria, thrombosis) and serious infusion reactions were not seen. The dose of everolimus was reduced in 4 patients due to rash (n = 2, dose reduction within the first cycle) and fatigue (n = 1, dose reduction in cycle 4); in one patient the reason for dose reduction on cycle 6 day 15 was not reported. Carotuximab was dose reduced in 1 patient on cycle 4 day 15 due to gingival hemorrhage. Eleven patients (73%) completed protocol therapy. Reasons for discontinuation included (all, n = 1) intercurrent illness, noncompliance, progressive disease (all taken off study on cycle 2); and development of anasarca/generalized edema (taken off study on cycle 4; association with the investigational therapy could not be determined).
Pharmacokinetics
Letrozole was rapidly absorbed from the gastrointestinal tract (maximum concentration (Cmax) reached within 2 h). With repeated daily administration, Cmax and the area under the concentration–time curve (AUC) increased by 5.5- and 2.4-fold, respectively, consistent with accumulation [34] (supplementary table 1). The measured concentrations of letrozole (which reaches steady state in 2–6 weeks [35]) on cycle 2 day 1 were consistently higher and more variable as compared with the predicted concentrations indicating non-linear pharmacokinetics (Fig. 1B).
PK analyses of everolimus were consistent with significantly lower maximum and trough concentrations (Ctrough) from those previously reported (Cmax: 5 mg, 2.63 ng/mL; 10 mg, 5.38 ng/mL vs. 32 and 61 ng/mL, respectively [36]; Ctrough: 5 mg, 0.354 (estimated) ng/mL; 10 mg, 0.323 (estimated) ng/mL vs. 5.4 and 13.2 ng/mL, respectively [36], Fig. 1C and supplementary table 1). Similarly, the AUC’s at steady state were calculated to be at 1/10 of the AUC’s previously reported [36, 37]. The half-life of everolimus was similar in the 2 dose levels (5 mg: 13.4 h, 10 mg: 11.8 h), and significantly lower from the 30 h previously reported [36]. There was a good concordance between the estimated and actual steady-state concentrations (supplementary Fig. 1A). No PK interactions between letrozole and everolimus are known to occur [37] indicating that carotuximab accelerated significantly the extravasation of everolimus (estimated volume of distribution exceeded 4000 and 5000 L for 5 and 10 mg of everolimus, respectively) as well as its clearance to the point that predose everolimus concentrations dropped below the lower limit of quantitation.
Serum concentrations of carotuximab known to saturate CD105 receptors (200 ng/mL) were rapidly achieved and consistently maintained (supplementary Fig. 1B). With dose escalation from 3 to 15 mg/kg, AUC and half-life increased supraproportionally while clearance decreased by a similar magnitude probably due to target saturation (supplementary table 2). PK simulations using a two-compartment model suggest accumulation of carotuximab in the extravascular volume of distribution (Fig. 1D).
Pharmacodynamics and antitumor activity
All patients underwent definitive surgery with curative intent. One patient with a basal-like intrinsic molecular subtype (see below) progressed clinically and radiographically and she transitioned to preoperative chemotherapy. All patients had residual disease on surgical pathology; pCR was achieved in one of two sites in a patient with multifocal disease. To capture treatment effect, we also assessed pathologic response by means of Residual Cancer Burden (RCB) scoring [38] (Fig. 2A). Among the remaining tumors, moderate treatment effect (RCB-II) was seen in 14 (14/16, 87.5%; 95% CI 61.65–98.45) while residual disease was extensive in 2 (RCB-III, 12.5%; 95% CI 1.55–38.35). Downstaging from stage 2/3 to 0/1 was achieved in 4/17 tumors (23.5%; 95% CI 6.81–49.9), stage did not change in 8/17 (47.1%; 95% CI 23–72.2), and 4/17 tumors were upstaged (23.5%; 95% CI 6.81–49.9) (Fig. 2B). Of the 4 tumors that were upstaged, 3 were invasive lobular and 1 invasive mammary carcinomas. Given the diffuse growth pattern and lack of desmoplastic reaction in invasive lobular carcinoma, this finding may reflect underestimation of the extent of the original disease rather than interval tumor growth [10]. Representative radiographic responses are shown in Fig. 2C and supplementary Fig. 2. The expression of the proliferation marker Ki-67 also decreased with the investigational therapy and its changes aligned with intrinsic subtype changes or lack thereof (Fig. 2D).
Antitumor activity. A Barplot of the RCB scores for the individual tumors. The patient with basal-like intrinsic subtype who transitioned to chemotherapy was excluded. B Piechart of change in stage (clinical stage at diagnosis – pathologic stage at surgery) with the investigational regimen. C Representative imaging response on MRI. D Pre- and on-treatment (cycle 2 day 1) changes of Ki67. Note the upregulation of Ki67 in the patient with the basal-like intrinsic subtype
To characterize gene expression changes induced by the investigational therapy, we performed genomic analyses on a NanoString nCounter platform (Breast Cancer 360 panel) of paired macrodissected tumor samples obtained at the time of diagnosis and on cycle 2 day 1. We successfully tested and analyzed 10 paired samples; one on-treatment sample did not pass quality control.
By PAM50 Molecular Subtype Signature, at diagnosis, 6 patients had luminal B, 3 patients had luminal A, and 1 patient had basal-like intrinsic subtype (Fig. 3A, supplementary Fig. 3 and 4). Of the 6 patients with luminal B breast cancer at diagnosis, 5 converted to luminal A on cycle 2 day 1 yielding a “molecular downstaging” rate of 83.3% [39]. Excluding the patient with the basal-like intrinsic subtype who had a distinctive gene expression profile (Fig. 3B and supplementary Fig. 5A), differential gene expression analyses between paired pre- and on-treatment samples show significant downregulation in the expression of genes involved in or regulating cell division (39/53 genes significantly downregulated at a p adjusted for multiple comparisons by Bergamini–Hochberg < 0.001; enrichment score p 8.89 × 10–23/False Discovery Rate 2.73 × 10–22) (Fig. 3C, D). The second most overrepresented functional category of downregulated genes, included genes involved in DNA repair most notably BRCA1 (enrichment score p 2.08 × 10–7/False Discovery Rate 3.21 × 10–6). A possible explanation for the upregulation and attendant treatment-related downregulation of these genes, may rely on fact that the ER induces transcriptional stress via R-loop formation, i.e. 3-stranded nucleic acid structures comprising a DNA:RNA duplex and a displaced single-stranded DNA [40]. Persistent R-loop accumulation can compromise genomic integrity which in turn, is mitigated by the BRCA1/2 genes [41, 42].
Correlative Studies and Relapse-free Survival. A Distribution of the intrinsic molecular subtypes at diagnosis and after one cycle of the investigational therapy. B Principle component analysis of all samples (pre- and on-treatment) with successful gene expression profiling by Nanostring. Note the distinction of the tumor with the basal-like intrinsic subtype from the cluster with the luminal tumors. Note also the proximity of the pre (circle) and on-treatment (triangle) points for the basal-like intrinsic subtype suggestive of lack of changes in gene expression with treatment unlike the luminal counterparts. C Volcano plot of the differentially expressed genes in the luminal cancers following one cycle of the investigational therapy. Differentially expressed genes at p-adjusted < 0.001 (above dashed horizontal line) are indicated in red; differentially expressed genes at p-adjusted < 0.001 involved in cell proliferation and DNA repair indicated in darkorange and aquamarine, respectively. D Bubble plot of the ontology of genes whose expression was downregulated with treatment p < 0.001 (adjusted by the Bergamini–Hochberg method for multiple comparisons). Bubbles are color coded by ontology category. The size of each bubble is proportionate to the number of genes falling into this category. Gene ontologies are plotted against the significance of gene-term enrichment (p-value, y-axis). E Immunohistochemistry for CD105 assessed in the vascular, nuclear, cytoplasmic, and membranous compartment on cycle 1 day 1 (C1D1) and cycle 2 day 1 (C2D1). F Kaplan–Meier curve of the relapse-free survival
The presence of non-luminal intrinsic subtypes in the immunophenotypically HR+/Her2− subgroup has been well recognized [43, 44]. By comparison to the luminal tumors, overexpression of genes that typify a basal-like subtype (cytokeratin 5 [45, 46], BCL11A and FOXC1 [47, 48]) or are associated with resistance to endocrine therapies (STAT1 [49], cyclin E [50]), and the lower expression of ESR1 and PGR may underpin the absence of gene expression changes and clinical response to the investigational therapy (supplementary Fig. 5 A and B).
CD105 (endoglin) was predominantly expressed in the vessels (Fig. 3E). CD105 expression, as assessed by immunohistochemistry, did not change significantly with the investigational therapy. The lack of change in CD105 expression may be due to early on-treatment CD105 evaluation because decreases in signal enhancement between baseline and cycle 4 day 1 breast MRIs were observed (Fig. 2C and supplementary Fig. 2). The absence of change may also be consistent with the mechanism of action of carotuximab whereby the antibody accelerates the cleavage and shedding of endoglin by coupling it with the membrane-anchored matrix-metalloproteinase 14 without changing the expression of the endoglin itself [51].
Semiquantitative assessment of the residual tumors for stromal tumor infiltrating lymphocytes (TILs) was consistent with no or minimal levels of TILs in 15/17 tumors (patient who progressed and transitioned to chemotherapy excluded; 88.2%; 95% CI 63.6–98.5) [52, 53].
The protocol left postoperative treatment decisions at the discretion of the treating physicians. None of 4 patients whose tumors downstaged with the investigational therapy received adjuvant chemotherapy; 4/7 (57%) and 2/3 (66%) patients whose tumors did not change stage or were upstaged, respectively, received adjuvant chemotherapy. With a median follow up of 45.6 months after surgery for curative intent (53.4 months from cycle 1 day 1), 3 patients relapsed (Fig. 3F, supplementary Fig. 6). The patient with the basal-like intrinsic subtype experienced an early recurrence despite neoadjuvant chemotherapy. Among the 5 patients whose tumors converted from luminal B to luminal A, 2 received adjuvant chemotherapy. None of the remaining 3 patients experienced a recurrence (follow up, 24.1–50.4 months; median, 40.1).
Discussion
This study met its primary endpoint of determining the MTD, RP2D, and rates of adverse events associated with the combination of letrozole with everolimus and carotuximab ± goserelin. All agents were tolerated at their single-agent doses. The toxicity profile was dominated by carotuximab and phenocopied HHT. Despite the paucity of adverse events greater than grade 2, all patients experienced adverse events that accumulated over time leading to frequent dose reductions. One should acknowledge the excellent performance status of patients with newly diagnosed breast cancer as well as the fact that endocrine combinations with novel oral agents, particularly inhibitors of the cyclin dependent kinase 4 and 6 (CDK4/6), can yield similarly high rates of molecular downstaging with fewer toxicities [39].
Pharmacokinetic analyses, which constituted a secondary objective of the study, show that, despite the targeted nature of carotuximab and everolimus, interactions did arise. The accumulation of carotuximab in the extravascular space with successive administrations may explain the later-onset anemia (typically seen after cycle 3) and the rising frequency and severity of adverse events associated with carotuximab (headaches, fatigue, epistaxis, gingival hemorrhage, nasal congestion, and telangiectasias). Carotuximab also accelerated the biodistribution of everolimus which probably underpins a pharmacodynamic interaction, in terms of significant downregulation of a proliferative gene expression signature as well as causation of synergistic adverse events (fatigue, skin rash, gingival hemorrhage). The long interval required for letrozole to reach steady state and the accumulation with repeated dosing may explain the later-onset of adverse events typically associated with this agent.
In terms of assessing the antitumor activity of preoperative endocrine therapy and especially combinatorial regimens, uniform criteria for optimal patient selection, endpoints to capture the activity of preoperative endocrine therapy, and biomarkers to escalate or de-escalate therapy have not been adopted.
Our results and other trials [54] suggest that molecular criteria should be considered for patient selection in these trials. Luminal A tumors may respond to endocrine monotherapy; additional agents and chemotherapy can be spared [55]. Nonluminal HR+/Her2− tumors and especially basal-like have distinct molecular underpinnings and a response to endocrine therapy should not be expected [44]. In our study, the most meaningful reductions in proliferation and gene expression changes were seen in luminal B tumors.
Recognizing that the achievement of pCR is an endpoint that does not align with the nature of luminal breast cancer and conventional pathologic criteria do not capture the gene expression changes induced by preoperative endocrine therapy, other, mostly proliferation-based biomarkers have been used [44, 53, 56,57,58]. Reductions in Ki67 with preoperative endocrine therapy have been associated with excellent long-term outcomes sparing chemotherapy [44, 54]. However, how well reductions in Ki67 can prognosticate patients with higher genomic risk disease who receive preoperative combinatorial endocrine regimens remains an open question: postoperative treatment decisions were left to the physicians’ discretion, long-term outcomes have not been reported or captured, and Ki67 can rebound upon treatment discontinuation [53, 57]. Regarding MammaPrint, the appealing concept of molecular downstaging, i.e. conversion from luminal B to PAM50 low-risk-of relapse disease, has been introduced [39]. Its ability to predict relapse or identify patients who can safely forego adjuvant chemotherapy and, more so, whether it has the same value irrespective of the regimen that induced it, remain to be evaluated. Although the administration of postoperative chemotherapy at physicians’ discretion precludes a clean readout of the long-term impact of the investigational therapy, our results provide clues that patients who achieve molecular downstaging have low risk of early relapse and potentially can be spared chemotherapy.
In conclusion, letrozole, everolimus, and carotuximab ± goserelin were tolerated in combination at their single-agent doses. Escalating the number of targeted agents in combinatorial preoperative regimens, may enhance the toxicities due to unaccounted interactions while the respective increments in antitumor activity may remain elusive.
Data availability
The datasets generated during and/or analyzed during the current study are available in the Mendeley repository (https://data.mendeley.com/datasets/kxyb98kynn/draft?a=fc195a30-f79c-48df-8006-9fc9e69f7474).
Abbreviations
- AUC:
-
Area under the concentration–time curve
- BCL11A:
-
BAF chromatin remodeling complex subunit BCL11A
- BRCA1:
-
BRCA1 DNA repair associated
- BRCA2:
-
BRCA2 DNA repair associated
- CD105:
-
Endoglin
- CDK4/6:
-
Cyclin-dependent kinase 4 and cyclin-dependent kinase 6
- C1D1:
-
Cycle 1 day 1
- C2D1:
-
Cycle 2 day 1
- CI:
-
Confidence interval
- Cmax:
-
Maximum concentration
- Ctrough:
-
Trough concentration
- DLT:
-
Dose-limiting toxicity
- DNA:
-
Deoxyribonucleic acid
- ECOG:
-
Eastern cooperative oncology group
- ER/ESR1:
-
Estrogen receptor
- FOXC1:
-
Forkhead box C1
- Her2:
-
Epidermal growth factor receptor-2
- HHT:
-
Hereditary hemorrhagic telangiectasia
- HR:
-
Hormone receptor
- IV:
-
Intravenous
- kg:
-
Kilogram
- mg:
-
Milligram
- µL:
-
Microliter
- MRI:
-
Magnetic resonance imaging
- MRM:
-
Multiple reaction monitoring
- MTD:
-
Maximum tolerated dose
- mTOR:
-
Mammalian target of rapamycin
- ng:
-
Nanogram
- pCR:
-
Pathologic complete remission
- PK:
-
Pharmacokinetic
- PO:
-
Per os (oral)
- RCB:
-
Residual cancer burden
- PR/PGR:
-
Progesterone receptor
- RECIST:
-
Response evaluation criteria in solid tumors
- RNA:
-
Ribonucleic acid
- RP2D:
-
Recommended phase 2 dose
- SC:
-
Subcutaneously
- SAE:
-
Serious adverse event
- STAT1:
-
Signal transducer and activator of transcription 1
- TGF-β:
-
Transforming growth factor-beta
- TILs:
-
Tumor infiltrating lymphocytes
- TRC105:
-
Carotuximab
- VEGF:
-
Vascular endothelial growth factor
- vs.:
-
Versus
References
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE Jr, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384:164–172. https://doi.org/10.1016/S0140-6736(13)62422-8
Spring LM, Fell G, Arfe A, Sharma C, Greenup R, Reynolds KL, Smith BL, Alexander B, Moy B, Isakoff SJ, Parmigiani G, Trippa L, Bardia A (2020) Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin Cancer Res 26:2838–2848. https://doi.org/10.1158/1078-0432.CCR-19-3492
Spring LM, Gupta A, Reynolds KL, Gadd MA, Ellisen LW, Isakoff SJ, Moy B, Bardia A (2016) Neoadjuvant endocrine therapy for estrogen receptor-positive breast cancer: a systematic review and meta-analysis. JAMA Oncol 2:1477–1486. https://doi.org/10.1001/jamaoncol.2016.1897
Cataliotti L, Buzdar AU, Noguchi S, Bines J, Takatsuka Y, Petrakova K, Dube P, de Oliveira CT (2006) Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: the pre-operative “arimidex” compared to tamoxifen (PROACT) trial. Cancer 106:2095–2103. https://doi.org/10.1002/cncr.21872
Eiermann W, Paepke S, Appfelstaedt J, Llombart-Cussac A, Eremin J, Vinholes J, Mauriac L, Ellis M, Lassus M, Chaudri-Ross HA, Dugan M, Borgs M (2001) Preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann Oncol 12:1527–1532. https://doi.org/10.1023/a:1013128213451
Ellis MJ, Coop A, Singh B, Tao Y, Llombart-Cussac A, Janicke F, Mauriac L, Quebe-Fehling E, Chaudri-Ross HA, Evans DB, Miller WR (2003) Letrozole inhibits tumor proliferation more effectively than tamoxifen independent of HER1/2 expression status. Cancer Res 63:6523–6531
Ellis MJ, Tao Y, Luo J, A’Hern R, Evans DB, Bhatnagar AS, Chaudri Ross HA, von Kameke A, Miller WR, Smith I, Eiermann W, Dowsett M (2008) Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 100:1380–1388. https://doi.org/10.1093/jnci/djn309
Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A, Blohmer JU, Ashley SE, Francis S, Boeddinghaus I, Walsh G (2005) Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the immediate preoperative anastrozole, tamoxifen, or combined with tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 23:5108–5116. https://doi.org/10.1200/JCO.2005.04.005
Forero-Torres A, Saleh MN, Galleshaw JA, Jones CF, Shah JJ, Percent IJ, Nabell LM, Carpenter JT, Falkson CI, Krontiras H, Urist MM, Bland KI, De Los Santos JF, Meredith RF, Caterinicchia V, Bernreuter WK, O’Malley JP, Li Y, LoBuglio AF (2010) Pilot trial of preoperative (neoadjuvant) letrozole in combination with bevacizumab in postmenopausal women with newly diagnosed estrogen receptor- or progesterone receptor-positive breast cancer. Clin Breast Cancer 10:275–280. https://doi.org/10.3816/CBC.2010.n.035
Vaklavas C, Roberts BS, Varley KE, Lin NU, Liu MC, Rugo HS, Puhalla S, Nanda R, Storniolo AM, Carey LA, Saleh MN, Li Y, Delossantos JF, Grizzle WE, LoBuglio AF, Myers RM, Forero-Torres A (2020) TBCRC 002: a phase II, randomized, open-label trial of preoperative letrozole with or without bevacizumab in postmenopausal women with newly diagnosed stage 2/3 hormone receptor-positive and HER2-negative breast cancer. Breast Cancer Res 22:22. https://doi.org/10.1186/s13058-020-01258-x
Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366:520–529. https://doi.org/10.1056/NEJMoa1109653
Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, Campone M, Kubista E, Greil R, Bianchi G, Steinseifer J, Molloy B, Tokaji E, Gardner H, Phillips P, Stumm M, Lane HA, Dixon JM, Jonat W, Rugo HS (2009) Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol 27:2630–2637. https://doi.org/10.1200/JCO.2008.18.8391
Yamnik RL, Digilova A, Davis DC, Brodt ZN, Murphy CJ, Holz MK (2009) S6 kinase 1 regulates estrogen receptor alpha in control of breast cancer cell proliferation. J Biol Chem 284:6361–6369. https://doi.org/10.1074/jbc.M807532200
Yamnik RL, Holz MK (2010) mTOR/S6K1 and MAPK/RSK signaling pathways coordinately regulate estrogen receptor alpha serine 167 phosphorylation. FEBS Lett 584:124–128. https://doi.org/10.1016/j.febslet.2009.11.041
Bockhorn M, Tsuzuki Y, Xu L, Frilling A, Broelsch CE, Fukumura D (2003) Differential vascular and transcriptional responses to anti-vascular endothelial growth factor antibody in orthotopic human pancreatic cancer xenografts. Clin Cancer Res 9:4221–4226
Davis DW, Inoue K, Dinney CP, Hicklin DJ, Abbruzzese JL, McConkey DJ (2004) Regional effects of an antivascular endothelial growth factor receptor monoclonal antibody on receptor phosphorylation and apoptosis in human 253J B-V bladder cancer xenografts. Cancer Res 64:4601–4610. https://doi.org/10.1158/0008-5472.CAN-2879-2
Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM, ten Dijke P (2004) Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J 23:4018–4028. https://doi.org/10.1038/sj.emboj.7600386
Fonsatti E, Altomonte M, Nicotra MR, Natali PG, Maio M (2003) Endoglin (CD105): a powerful therapeutic target on tumor-associated angiogenetic blood vessels. Oncogene 22:6557–6563. https://doi.org/10.1038/sj.onc.1206813
Minhajat R, Mori D, Yamasaki F, Sugita Y, Satoh T, Tokunaga O (2006) Endoglin (CD105) expression in angiogenesis of colon cancer: analysis using tissue microarrays and comparison with other endothelial markers. Virchows Arch 448:127–134. https://doi.org/10.1007/s00428-005-0062-8
Bertolino P, Deckers M, Lebrin F, ten Dijke P (2005) Transforming growth factor-beta signal transduction in angiogenesis and vascular disorders. Chest 128:585S-590S. https://doi.org/10.1378/chest.128.6_suppl.585S
Blanco FJ, Santibanez JF, Guerrero-Esteo M, Langa C, Vary CP, Bernabeu C (2005) Interaction and functional interplay between endoglin and ALK-1, two components of the endothelial transforming growth factor-beta receptor complex. J Cell Physiol 204:574–584. https://doi.org/10.1002/jcp.20311
Dallas NA, Samuel S, Xia L, Fan F, Gray MJ, Lim SJ, Ellis LM (2008) Endoglin (CD105): a marker of tumor vasculature and potential target for therapy. Clin Cancer Res 14:1931–1937. https://doi.org/10.1158/1078-0432.CCR-07-4478
Rosen LS, Hurwitz HI, Wong MK, Goldman J, Mendelson DS, Figg WD, Spencer S, Adams BJ, Alvarez D, Seon BK, Theuer CP, Leigh BR, Gordon MS (2012) A phase I first-in-human study of TRC105 (anti-endoglin antibody) in patients with advanced cancer. Clin Cancer Res 18:4820–4829. https://doi.org/10.1158/1078-0432.CCR-12-0098
Takahashi N, Haba A, Matsuno F, Seon BK (2001) Antiangiogenic therapy of established tumors in human skin/severe combined immunodeficiency mouse chimeras by anti-endoglin (CD105) monoclonal antibodies, and synergy between anti-endoglin antibody and cyclophosphamide. Cancer Res 61:7846–7854
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28:2784–2795
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997–4013. https://doi.org/10.1200/JCO.2013.50.9984
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Apolo AB, Karzai FH, Trepel JB, Alarcon S, Lee S, Lee MJ, Tomita Y, Cao L, Yu Y, Merino MJ, Madan RA, Parnes HL, Steinberg SM, Rodriguez BW, Seon BK, Gulley JL, Arlen PM, Dawson NA, Figg WD, Dahut WL (2017) A phase II clinical trial of TRC105 (anti-endoglin antibody) in adults with advanced/metastatic urothelial carcinoma. Clin Genitourin Cancer 15:77–85. https://doi.org/10.1016/j.clgc.2016.05.010
Choueiri TK, Michaelson MD, Posadas EM, Sonpavde GP, McDermott DF, Nixon AB, Liu Y, Yuan Z, Seon BK, Walsh M, Jivani MA, Adams BJ, Theuer CP (2018) An open label phase Ib dose escalation study of TRC105 (anti-endoglin antibody) with axitinib in patients with metastatic renal cell carcinoma. Oncologist 24:202–210. https://doi.org/10.1634/theoncologist.2018-0299
Dorff TB, Longmate JA, Pal SK, Stadler WM, Fishman MN, Vaishampayan UN, Rao A, Pinksi JK, Hu JS, Quinn DI, Lara PN Jr (2017) Bevacizumab alone or in combination with TRC105 for patients with refractory metastatic renal cell cancer. Cancer 123:4566–4573. https://doi.org/10.1002/cncr.30942
Duffy AG, Ma C, Ulahannan SV, Rahma OE, Makarova-Rusher O, Cao L, Yu Y, Kleiner DE, Trepel J, Lee MJ, Tomita Y, Steinberg SM, Heller T, Turkbey B, Choyke PL, Peer CJ, Figg WD, Wood BJ, Greten TF (2017) Phase I and preliminary phase II study of TRC105 in combination with sorafenib in hepatocellular carcinoma. Clin Cancer Res 23:4633–4641. https://doi.org/10.1158/1078-0432.CCR-16-3171
Gordon MS, Robert F, Matei D, Mendelson DS, Goldman JW, Chiorean EG, Strother RM, Seon BK, Figg WD, Peer CJ, Alvarez D, Adams BJ, Theuer CP, Rosen LS (2014) An open-label phase Ib dose-escalation study of TRC105 (anti-endoglin antibody) with bevacizumab in patients with advanced cancer. Clin Cancer Res 20:5918–5926. https://doi.org/10.1158/1078-0432.CCR-14-1143
Rokhlin OW, Cohen MB, Kubagawa H, Letarte M, Cooper MD (1995) Differential expression of endoglin on fetal and adult hematopoietic cells in human bone marrow. J Immunol 154:4456–4465
Buzdar AU, Robertson JF, Eiermann W, Nabholtz JM (2002) An overview of the pharmacology and pharmacokinetics of the newer generation aromatase inhibitors anastrozole, letrozole, and exemestane. Cancer 95:2006–2016. https://doi.org/10.1002/cncr.10908
Bhatnagar AS (2007) The discovery and mechanism of action of letrozole. Breast Cancer Res Treat 105(Suppl 1):7–17. https://doi.org/10.1007/s10549-007-9696-3
O’Donnell A, Faivre S, Burris HA 3rd, Rea D, Papadimitrakopoulou V, Shand N, Lane HA, Hazell K, Zoellner U, Kovarik JM, Brock C, Jones S, Raymond E, Judson I (2008) Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol 26:1588–1595. https://doi.org/10.1200/JCO.2007.14.0988
Awada A, Cardoso F, Fontaine C, Dirix L, De Greve J, Sotiriou C, Steinseifer J, Wouters C, Tanaka C, Zoellner U, Tang P, Piccart M (2008) The oral mTOR inhibitor RAD001 (everolimus) in combination with letrozole in patients with advanced breast cancer: results of a phase I study with pharmacokinetics. Eur J Cancer 44:84–91. https://doi.org/10.1016/j.ejca.2007.10.003
Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, Assad L, Poniecka A, Hennessy B, Green M, Buzdar AU, Singletary SE, Hortobagyi GN, Pusztai L (2007) Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 25:4414–4422. https://doi.org/10.1200/JCO.2007.10.6823
Prat A, Saura C, Pascual T, Hernando C, Munoz M, Pare L, Gonzalez Farre B, Fernandez PL, Galvan P, Chic N, Gonzalez Farre X, Oliveira M, Gil-Gil M, Arumi M, Ferrer N, Montano A, Izarzugaza Y, Llombart-Cussac A, Bratos R, Gonzalez Santiago S, Martinez E, Hoyos S, Rojas B, Virizuela JA, Ortega V, Lopez R, Celiz P, Ciruelos E, Villagrasa P, Gavila J (2020) Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol 21:33–43. https://doi.org/10.1016/S1470-2045(19)30786-7
Stork CT, Bocek M, Crossley MP, Sollier J, Sanz LA, Chedin F, Swigut T, Cimprich KA (2016) Co-transcriptional R-loops are the main cause of estrogen-induced DNA damage. Elife. https://doi.org/10.7554/eLife.17548
Bhatia V, Barroso SI, Garcia-Rubio ML, Tumini E, Herrera-Moyano E, Aguilera A (2014) BRCA2 prevents R-loop accumulation and associates with TREX-2 mRNA export factor PCID2. Nature 511:362–365. https://doi.org/10.1038/nature13374
Hatchi E, Skourti-Stathaki K, Ventz S, Pinello L, Yen A, Kamieniarz-Gdula K, Dimitrov S, Pathania S, McKinney KM, Eaton ML, Kellis M, Hill SJ, Parmigiani G, Proudfoot NJ, Livingston DM (2015) BRCA1 recruitment to transcriptional pause sites is required for R-loop-driven DNA damage repair. Mol Cell 57:636–647. https://doi.org/10.1016/j.molcel.2015.01.011
Cejalvo JM, Pascual T, Fernandez-Martinez A, Braso-Maristany F, Gomis RR, Perou CM, Munoz M, Prat A (2018) Clinical implications of the non-luminal intrinsic subtypes in hormone receptor-positive breast cancer. Cancer Treat Rev 67:63–70. https://doi.org/10.1016/j.ctrv.2018.04.015
Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A, Parker JS, Luo J, DeSchryver K, Allred DC, Esserman LJ, Unzeitig GW, Margenthaler J, Babiera GV, Marcom PK, Guenther JM, Watson MA, Leitch M, Hunt K, Olson JA (2011) Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype–ACOSOG Z1031. J Clin Oncol 29:2342–2349. https://doi.org/10.1200/JCO.2010.31.6950
Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, Perou CM, Nielsen TO (2008) Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res 14:1368–1376. https://doi.org/10.1158/1078-0432.CCR-07-1658
Foulkes WD, Smith IE, Reis-Filho JS (2010) Triple-negative breast cancer. N Engl J Med 363:1938–1948. https://doi.org/10.1056/NEJMra1001389
Khaled WT, Choon Lee S, Stingl J, Chen X, Raza Ali H, Rueda OM, Hadi F, Wang J, Yu Y, Chin SF, Stratton M, Futreal A, Jenkins NA, Aparicio S, Copeland NG, Watson CJ, Caldas C, Liu P (2015) BCL11A is a triple-negative breast cancer gene with critical functions in stem and progenitor cells. Nat Commun 6:5987. https://doi.org/10.1038/ncomms6987
Han B, Bhowmick N, Qu Y, Chung S, Giuliano AE, Cui X (2017) FOXC1: an emerging marker and therapeutic target for cancer. Oncogene 36:3957–3963. https://doi.org/10.1038/onc.2017.48
Anurag M, Zhu M, Huang C, Vasaikar S, Wang J, Hoog J, Burugu S, Gao D, Suman V, Zhang XH, Zhang B, Nielsen T, Ellis MJ (2019) immune checkpoint profiles in luminal B breast cancer (alliance). J Natl Cancer Inst. https://doi.org/10.1093/jnci/djz213
Doostan I, Karakas C, Kohansal M, Low KH, Ellis MJ, Olson JA Jr, Suman VJ, Hunt KK, Moulder SL, Keyomarsi K (2017) Cytoplasmic cyclin E mediates resistance to aromatase inhibitors in breast cancer. Clin Cancer Res 23:7288–7300. https://doi.org/10.1158/1078-0432.CCR-17-1544
Kumar S, Pan CC, Bloodworth JC, Nixon AB, Theuer C, Hoyt DG, Lee NY (2014) Antibody-directed coupling of endoglin and MMP-14 is a key mechanism for endoglin shedding and deregulation of TGF-beta signaling. Oncogene 33:3970–3979. https://doi.org/10.1038/onc.2013.386
Waks AG, Stover DG, Guerriero JL, Dillon D, Barry WT, Gjini E, Hartl C, Lo W, Savoie J, Brock J, Wesolowski R, Li Z, Damicis A, Philips AV, Wu Y, Yang F, Sullivan A, Danaher P, Brauer HA, Osmani W, Lipschitz M, Hoadley KA, Goldberg M, Perou CM, Rodig S, Winer EP, Krop IE, Mittendorf EA, Tolaney SM (2019) The immune microenvironment in hormone receptor-positive breast cancer before and after preoperative chemotherapy. Clin Cancer Res 25:4644–4655. https://doi.org/10.1158/1078-0432.CCR-19-0173
Hurvitz SA, Martin M, Press MF, Chan D, Fernandez-Abad M, Petru E, Rostorfer R, Guarneri V, Huang CS, Barriga S, Wijayawardana S, Brahmachary M, Ebert PJ, Hossain A, Liu J, Abel A, Aggarwal A, Jansen VM, Slamon DJ (2020) Potent cell-cycle inhibition and upregulation of immune response with abemaciclib and anastrozole in neoMONARCH, phase II neoadjuvant study in HR(+)/HER2(-) breast cancer. Clin Cancer Res 26:566–580. https://doi.org/10.1158/1078-0432.CCR-19-1425
Nitz UA, Gluz O, Kummel S, Christgen M, Braun M, Aktas B, Ludtke-Heckenkamp K, Forstbauer H, Grischke EM, Schumacher C, Darsow M, Krauss K, Nuding B, Thill M, Potenberg J, Uleer C, Warm M, Fischer HH, Malter W, Hauptmann M, Kates RE, Graser M, Wurstlein R, Shak S, Baehner F, Kreipe HH, Harbeck N (2022) Endocrine therapy response and 21-gene expression assay for therapy guidance in HR+/HER2− early breast cancer. J Clin Oncol 40:2557–2567. https://doi.org/10.1200/JCO.21.02759
Nitz U, Gluz O, Christgen M, Kates RE, Clemens M, Malter W, Nuding B, Aktas B, Kuemmel S, Reimer T, Stefek A, Lorenz-Salehi F, Krabisch P, Just M, Augustin D, Liedtke C, Chao C, Shak S, Wuerstlein R, Kreipe HH, Harbeck N (2017) Reducing chemotherapy use in clinically high-risk, genomically low-risk pN0 and pN1 early breast cancer patients: five-year data from the prospective, randomised phase 3 West German Study Group (WSG) PlanB trial. Breast Cancer Res Treat 165:573–583. https://doi.org/10.1007/s10549-017-4358-6
Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, A’Hern R, Salter J, Detre S, Hills M, Walsh G, Group IT (2007) Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst 99:167–170. https://doi.org/10.1093/jnci/djk020
Ma CX, Gao F, Luo J, Northfelt DW, Goetz M, Forero A, Hoog J, Naughton M, Ademuyiwa F, Suresh R, Anderson KS, Margenthaler J, Aft R, Hobday T, Moynihan T, Gillanders W, Cyr A, Eberlein TJ, Hieken T, Krontiras H, Guo Z, Lee MV, Spies NC, Skidmore ZL, Griffith OL, Griffith M, Thomas S, Bumb C, Vij K, Bartlett CH, Koehler M, Al-Kateb H, Sanati S, Ellis MJ (2017) NeoPalAna: neoadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor-positive breast cancer. Clin Cancer Res 23:4055–4065. https://doi.org/10.1158/1078-0432.CCR-16-3206
Johnston S, Puhalla S, Wheatley D, Ring A, Barry P, Holcombe C, Boileau JF, Provencher L, Robidoux A, Rimawi M, McIntosh SA, Shalaby I, Stein RC, Thirlwell M, Dolling D, Morden J, Snowdon C, Perry S, Cornman C, Batten LM, Jeffs LK, Dodson A, Martins V, Modi A, Osborne CK, Pogue-Geile KL, Cheang MCU, Wolmark N, Julian TB, Fisher K, MacKenzie M, Wilcox M, Huang Bartlett C, Koehler M, Dowsett M, Bliss JM, Jacobs SA (2019) Randomized phase II study evaluating palbociclib in addition to letrozole as neoadjuvant therapy in estrogen receptor-positive early breast cancer: PALLET trial. J Clin Oncol 37:178–189. https://doi.org/10.1200/JCO.18.01624
Betts A, Keunecke A, van Steeg TJ, van der Graaf PH, Avery LB, Jones H, Berkhout J (2018) Linear pharmacokinetic parameters for monoclonal antibodies are similar within a species and across different pharmacological targets: a comparison between human, cynomolgus monkey and hFcRn Tg32 transgenic mouse using a population-modeling approach. MAbs 10:751–764. https://doi.org/10.1080/19420862.2018.1462429
Romero-Moreno R, Curtis KJ, Coughlin TR, Miranda-Vergara MC, Dutta S, Natarajan A, Facchine BA, Jackson KM, Nystrom L, Li J, Kaliney W, Niebur GL, Littlepage LE (2019) The CXCL5/CXCR2 axis is sufficient to promote breast cancer colonization during bone metastasis. Nat Commun 10:4404. https://doi.org/10.1038/s41467-019-12108-6
Leontovich AA, Jalalirad M, Salisbury JL, Mills L, Haddox C, Schroeder M, Tuma A, Guicciardi ME, Zammataro L, Gambino MW, Amato A, Di Leonardo A, McCubrey J, Lange CA, Liu M, Haddad T, Goetz M, Boughey J, Sarkaria J, Wang L, Ingle JN, Galanis E, D’Assoro AB (2018) NOTCH3 expression is linked to breast cancer seeding and distant metastasis. Breast Cancer Res 20:105. https://doi.org/10.1186/s13058-018-1020-0
Acknowledgements
The authors wish to acknowledge Kimberly LaMaster (study coordinator), Deborah Della Manna (laboratory technician), the patients and their families who participated in this clinical trial.
Funding
This study was supported by O’Neal Comprehensive Cancer Center at the University of Alabama at Birmingham. Correlative studies were supported by Young Supporters Board Faculty Development Grant (to C.V.). Support in the form of provision of the investigational agents was provided by Novartis and TRACON Pharmaceuticals. The funding agencies were not involved in the design of the study and collection, analysis, or in the interpretation of data and writing of the manuscript.
Author information
Authors and Affiliations
Contributions
Protocol writing: CV, AFT. Conception and design: CV, AFT. Provision of study materials or patients: CV, EMSR, AME, KJR, CPT, EPA, ESY, WEG, AFT. Collection and assembly of data: all authors. Data analysis and interpretation: all authors. Manuscript writing: CV, AFT. Final approval of manuscript: all authors.
Corresponding author
Ethics declarations
Competing interests
C.V. consulting or advisory board: Guidepoint, Novartis, SeaGen, Daiichi Sankyo, Genentech (unpaid); research funding (paid to the institution): Pfizer, SeaGen, H3 Biomedicine/Eisai, AstraZeneca, CytomX; employment: Flatiron (wife); Other: Gilead, AstraZeneca. Open payments link: https://openpaymentsdata.cms.gov/physician/1306968; A.F.T. received support from Genentech to conduct clinical and laboratory research (paid to the University of Alabama at Birmingham). A.F.T. is currently an employee of SeaGen. The remaining authors have no conflict of interest to disclose.
Ethical approval
The protocol was reviewed and approved by the Institutional Review Board of the University of Alabama at Birmingham, and the study followed the Declaration of Helsinki and Good Clinical Practice guidelines. All patients gave informed consent. This study is registered on the clinical trial website of the US National Cancer Institute (https://clinicaltrials.gov/ct2/show/NCT02520063).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vaklavas, C., Stringer-Reasor, E.M., Elkhanany, A.M. et al. A phase I/II study of preoperative letrozole, everolimus, and carotuximab in stage 2 and 3 hormone receptor-positive and Her2-negative breast cancer. Breast Cancer Res Treat 198, 217–229 (2023). https://doi.org/10.1007/s10549-023-06864-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-06864-9