Abstract
Purpose
The Ontario Breast Screening Program (OBSP) offers free screening mammograms every 2 years, to women aged 50–74. Study objectives were to determine demographic characteristics associated with the adherence to OBSP and if women screened in the OBSP have a lower stage at diagnosis than non-screened eligible women.
Methods
We used the Ontario cancer registry (OCR) to identify 48,927 women, aged 51–74 years, diagnosed with breast cancer between 2010 and 2017. These women were assigned as having undergone adherent screening (N = 26,108), non-adherent screening (N = 6546) or not-screened (N = 16,273) in the OBSP. We used multinomial logistic regression to investigate the demographic characteristics associated with screening behaviour, as well as the association between screening status and stage at diagnosis.
Results
Among women with breast cancer, those living in rural areas (versus the largest urban areas) had a lower odds of not being screened (odds ratio [OR] 0.73, 95% confidence interval [CI] 0.68, 0.78). Women in low-income (versus high-income) communities were more likely not to be screened (OR 1.42, 95% CI 1.33, 1.51). When stratified, the association between income and screening status only held in urban areas. Non-screened women were more likely to be diagnosed with stage II (OR 1.91, 95% CI 1.82, 2.01), III (OR 2.96, 95% CI 2.76, 3.17), or IV (OR 8.96, 95% CI 7.94, 10.12) disease compared to stage I and were less likely to be diagnosed with ductal carcinoma in situ (DCIS) (OR 0.91, 95% CI 0.84–0.98).
Conclusions
This study suggests that targeting OBSP recruitment efforts to lower income urban communities could increase screening rates. OBSP adherent women were more likely to be diagnosed with earlier stage disease, supporting the value of this initiative and those like it.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most commonly diagnosed malignancy in women worldwide, with one in eight women in Canada expected to develop the disease in their lifetime [1]. In the province of Ontario Canada, the Ontario Breast Screening Program (OBSP) provides breast cancer screening with mammography every 2 years to eligible women in the population (ages 50–74 years). Along with these age criteria, to be eligible, women must have no new breast cancer symptoms, personal history of breast cancer, breast implants, or history of mastectomy [2]. Annual screening is available for women who have a family history of breast or ovarian cancer, extremely dense breasts, or a history of high-risk lesions [2, 3]. In the year 2018, approximately 77% of all women eligible for breast cancer screening within and outside of the OBSP received a screening mammogram within the previous 30 months [3]. While it is good that nearly two-thirds of women in Ontario had received at least one mammogram during this interval, there is still a substantial proportion of screening-eligible women who may be under-screened or not screened at all. This is concerning given the evidence that breast cancer screening leads to the diagnosis of early-stage disease, [4, 5] which in turn is associated with lower breast cancer mortality [6].
Those who undergo breast cancer screening are known to differ from those that do not, based on some key demographic characteristics. For example, it has been shown that individuals living in a rural community [7] or having low-income socio-economic status [8] are less likely to participate in breast cancer screening in the United States and the Netherlands, respectively. Understanding the characteristics of individuals who do not get screened is essential to inform policies seeking to increase participation and adherence in breast screening programmes. This will ensure equitable access to, and sharing of, the benefits of such programmes.
Accordingly, this study had two main objectives. The first was to identify the characteristics associated with participation in the OBSP and the second is to determine if OBSP screening is indeed associated with a lower stage at diagnosis.
Methods
Study population
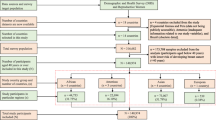
The retrospective cohort under investigation was identified through the Ontario Cancer Registry (OCR) and includes records for all women ages 51–74 years in Ontario diagnosed with a first primary breast cancer (invasive or ductal carcinoma in situ [DCIS]) between 2010 and 2017 (N = 53,627) (Fig. 1). Information on breast cancer screening was obtained from OBSP records and included 145,274 screening records spanning from 2006 to 2017. These records represented singular screening events from 43,953 unique patients (Fig. 1).
For women with a second primary breast cancer between 2010 and 2017 (N = 3,304), only information on their first breast cancer diagnosis in that time-period was included. An individual woman in the sample could have multiple screening events through routine participation in the OBSP. Symptomatic (diagnostic) breast cancer screening events (N = 8890) and any screening event for women with breast implants (because screening recommendations in the OBSP differ for women with breast implants, N = 170) were excluded. Women in the High Risk OBSP or those who were eligible for the High Risk OBSP (N = 842) were excluded, because these individuals follow different screening guidelines [3].
Screening behaviour
Adherent screeners were defined as those who had one OBSP mammogram at least once every 3 years for those eligible for biennial screening or once every 2 years for those on annual recall screening, starting at age 50 years. We provided women with a grace period of approximately 1 year for both annual and biennial screening to allow for variability due to scheduling. Conversely, women who had been diagnosed with breast cancer but had no record of screening within the OBSP were defined as non-screening individuals. Women who had at least one OBSP screen, but not according to OBSP guidelines, were classified as non-adherent screeners. Because the OBSP starts screening women at age 50 years, women diagnosed prior to age 51 years were not eligible for the current study so we could accurately determine their screening status (i.e. women diagnosed with breast cancer at age 51 years were classified as adherent screeners if screened at age 50 years, and a non-screener if not). After the application of all exclusion criteria, the final cohort included 48,927 individual women (91.2%) (Fig. 1).
Cancer stage and tumour characteristics
Data based on TNM stage guidelines were used to define cancer stage for this analysis. This classification guideline uses tumour size (T), number of surrounding lymph nodes with cancer (N), and cancer metastasis (M) to determine the stage of cancer at diagnosis. Within this dataset, stage zero cancers included any non-staged forms of breast cancer such as Paget’s disease or Phyllodes tumours [9, 10]. DCIS was classified in its own category. Missing data for cancer stage were classified as unknown cancer stage (N = 3565, 7.3%). Data for both estrogen (ER) and progesterone (PR) receptor status and human epidermal growth factor receptor 2 (HER2) status were also obtained from the OCR. The outcomes for these three hormone receptors were classified as positive, negative, or unknown (includes borderline status). If a woman was diagnosed with multi-focal or bilateral disease, characteristics of the highest stage tumour were used.
Patient characteristics
Data on an individual’s community size, urban, or rural setting and income were obtained from Ontario Health (Cancer Care Ontario). Statistics Canada’s definition of census metropolitan area (CMA) was used to categorize the residential community size [11]. A CMA is defined as urban areas of differing population size from under 100,000 people, 100,000–499,999, 500,000–1,499,999, or over 1,500,000. Individuals who lived outside of CMAs were defined as living in a rural community [11]. Before tax, neighbourhood income quintile was categorized as a three-category variable combining the three middle-income quintiles (middle, lower-middle, upper-middle) into a single middle-income level, while the lowest and the highest income quintiles were maintained as is [12]. All instances where community size or income were missing were coded as ‘Unknown’ (N = 743, 1.5%).
Statistical analyses
Standardized differences (SD) were used to compare patient characteristics (e.g. residential community size, neighbourhood income, age, and presence of a prior non-breast cancer (yes/no)), and tumour characteristics such as cancer stage, ER, PR, and HER2 receptor status between non-screeners, non-adherent screeners, and adherent screeners. A standardized difference greater than or equal to 0.1 was used to represent a meaningful difference between groups [13].
Multivariable adjusted multinomial logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CI) for the association between age, neighbourhood income, residential community size, and prior non-breast cancer with screening behaviour (non-screener or non-adherent screener versus adherent screener) in a mutually adjusted model. A second model was run exploring the potential interaction between community size and income quintile using a product term. This led to models being stratified by community size. Multinomial logistic regression (generalized logit) was used to examine the association between screening behaviour (adherent screener versus non-adherent screener or non-screener) and cancer stage at diagnosis (stage I–IV, DCIS, and non-staged cancer). Age, prior non-breast cancer, and residential community size were all treated as confounders for this analysis.
All tests were two-sided, and a p-value of less than 0.05 was considered statistically significant. Data cleaning and standardized differences were performed in R, and all model creation and diagnostics were performed in SAS Studio Version 9.4.
Results
The median age at breast cancer diagnosis was 63 years, with those who were screened at least once being slightly older than those who were not screened (SD = 0.23). Over a third of the cohort (38.4%) resided in a metropolitan area with a population of more than 1,500,000 people, while 12.1% of the cohort lived in a rural setting (Table 1). Most women also fell within the middle-income category (59.2%), with no difference in screening behaviours observed (SDs < 0.1) (Table 1).
Overall, stage I cancers were the most (40.0%, N = 19,587) and stage IV, the least (3.8%, n = 1,851) common stage at diagnosis in the cohort (Table 1). Of all the women diagnosed with breast cancer between 2010 and 2017, 53.4% were classified as being adherent screeners. In unadjusted analyses, the relative fraction of stage I cancers was larger among non-adherent and adherent screeners versus non-screeners (SD = 0.22). Correspondingly, non-screeners had a higher proportion of stage III (SD = 0.15) and IV (SD = 0.21) cancers. DCIS was more commonly diagnosed in adherent screeners compared to non-adherent and non-screening individuals (SD = 0.11).
The majority of cancers with available information on receptor status (ER: 78.9%, PR: 78.8%, HER2: 59.1%) were ER-positive (84.7%), PR-positive (74.5%), and HER2-negative (85.3%). A larger proportion of non-adherent screening women were HER2-negative (SD = 0.20) and a smaller proportion had unknown HER2 receptor status (SD = 0.21), but there was a similar proportion of women with HER2-positive cancers regardless of compliance (SD < 0.1) (Table 1).
Patient characteristics and screening behaviours
The odds of being a non-screener decreased with every 5-year increase in age (OR 0.93, 95% CI 0.92, 0.95) (Table 2). Compared to those living in an urban area of over 1,500,000 people, the odds of being a non-screener were significantly lower for those living in smaller urban areas, including communities with a population under 100,000 (OR 0.79, 95% CI 0.74, 0.85), between 100,000 and 499,999 people (OR 0.61, 95% CI 0.57, 0.64), and for those living in a rural area (OR 0.73, 95% CI 0.68, 0.78). An association between neighbourhood income and screening status was also observed. Specifically, the odds of being a non-screener compared to an adherent screener were significantly higher for those in the lowest and middle-income categories compared to the highest (OR 1.42, 95% CI 1.33, 1.51 and OR 1.08, 95% CI 1.03, 1.13, respectively).
The odds of being a non-adherent screener increased with every 5-year increase in age (OR 1.19, 95% CI 1.17, 1.22). Compared to those living in an urban area of over 1,500,000 people, the odds of being a non-screener were significantly lower for those living in smaller urban areas, including communities with a population under 100,000 (OR 0.79, 95% CI 0.71, 0.84), between 100,000 and 499,999 people (OR 0.84, 95% CI 0.78, 0.90), and for those living in a rural area (OR 0.78, 95% CI 0.72, 0.86). An association between neighbourhood income and screening status was also observed. Specifically, the odds of being a non-adherent screener compared to an adherent screener were significantly higher for those in the lowest income category (OR 1.25, 95% CI 1.15, 1.37), but not those in the middle-income category compared to the highest (OR 1.02, 95% CI 0.95, 1.09).
A significant interaction between community population size and income was observed (p = 0.04). In a model stratified by community population size, it was found that the effect of income on screening behaviour differed by community population size (Table 3). Specifically, lower income was associated with an increased odds of being a non-screener across all urban areas but was not associated with screening adherence among those living in a rural setting. For urban areas over 1,500,000 people, the odds of being a non-screener were highest for women in the lowest versus highest income category (OR 1.52, 95% CI 1.38, 1.67), and there were lower odds of being a non-screener for women in the middle versus lowest income category (OR 0.75, 95% CI 0.69, 0.81). This pattern was consistent across urban areas of different population sizes (Table 3).
A similar pattern was observed for women who were non-adherent screeners compared to adherent screeners. Specifically, lower income was associated with an increased odds of being a non-screener across all populations but was not associated with screening adherence among those in an urban area under 100,000. For rural areas, the odds of being a non-adherent screener were highest for women in the lowest versus highest income category (OR 1.42, 95% CI 1.09, 1.85), and there were lower odds of being a non-adherent screener for women in the middle versus lowest income category (OR 0.81, 95% CI 0.66, 0.99). This pattern was consistent across urban areas of different population sizes except for urban areas under 100,000 (Table 3).
Screening behaviour and tumour stage at diagnosis
Non-screeners had 9% lower odds of being diagnosed with DCIS compared to adherent screeners (OR 0.91, 95% CI 0.84, 0.98) (Table 4). Conversely, compared to stage I disease, the odds of being diagnosed with stage II (OR 1.91, 95% CI 1.82, 2.01), III (OR 2.96, 95% CI 2.76, 3.17), or IV (OR 8.96, 95% CI 7.94, 10.12) breast cancer were higher for non-screeners compared to adherent screeners and increased with increasing stage.
A similar pattern was observed among non-adherent screeners compared to adherent screeners. While non-adherent screeners had 4% non-significantly lower odds of being diagnosed with DCIS compared to adherent screeners (OR 0.96, 95% CI 0.88, 1.06), the odds of being diagnosed with stage II (OR 1.33, 95% CI 1.24, 1.42), III (OR 1.39, 95% CI 1.26, 1.55), or IV (OR 2.36, 95% CI 1.97, 2.82) breast cancer were significantly higher for non-adherent screeners compared to adherent screeners and increased with increasing stage.
Discussion
Overall, among women aged 51–74 years diagnosed with breast cancer in Ontario between 2010 and 2017, those that were screened according to OBSP guidelines were less likely to be diagnosed with later stage disease. In particular, non-screeners had an almost ninefold higher odds of being diagnosed with stage IV disease. However, as expected, these women were less likely to be diagnosed with DCIS (OR 0.91, 95% CI 0.84, 0.98). In addition, we found that Ontario women residing in urban areas with lower neighbourhood income had higher odds of being a non-screener.
Among women with breast cancer who were eligible to be screened in the OBSP (i.e. women ages 50–74 years), most were adherent screeners (n = 26,108, 53.4%), while a minority did not screen at all, or were exclusively screened outside of OBSP (n = 16,273, 33.3%). This is consistent with previous research [14]. Notably, very few women (N = 6,546, 13.4%) engaged in non-adherent screening (i.e. had at least one screening mammogram but did not follow OBSP guidelines). This suggests that individuals who get screened tend to participate fully in the programme, following OBSP guidelines. Focus on efforts to increase screening initiation in women living in larger urban areas with lower neighbourhood income will increase screening rates among screen-eligible women, at least among those later diagnosed with breast cancer. Further, increasing screening rates is likely to reduce the number of late-stage cancers diagnosed, improving cancer outcomes.
Prior work from the OBSP has shown that about 83% of OBSP women who initiated screening returned for a subsequent screen, and that this proportion consistently increased from 1992 to 2001 [14]. However, more recent trends suggest that screening retention has actually decreased in Ontario from 83% in 2012 to 77% in 2018 [3]. In particular, it has been shown that the odds of returning for a second screen are highest for those living in rural compared to urban areas [15]. However, when compared to the broader literature, the impact of rural versus urban living on screening behaviour is mixed. Some studies based in the United States have found that access to breast screening is more available in urban centres. This is reflected in the higher screening rates for women living in urban versus rural areas [7, 16, 17]. Other studies, in Australia and Croatia (both with publicly funded screening), have shown similar screening rates in women living in rural and urban settings [18, 19]. Overall, the literature suggests that access to screening services does tend to be lower for women living in rural areas [18, 20]. On the contrary, in our study, we found that women living in rural areas were less likely to be non-screeners. This suggests that having an organized, province-wide, publicly funded screening programme mitigates some of the rural–urban disparities in screening rates observed in other jurisdictions.
Data have also shown that those in the lowest neighbourhood income category tend to have higher odds of not being screened compared to those in the middle- or highest neighbourhood income categories [8, 21, 22]. Consistent with the evidence, in our study, individuals in lower- and middle-income neighbourhoods were more like to be non-screeners when compared to those in the highest income quintile. This is despite the fact that OBSP screening is publicly available, without the need for referral from primary care, and has no associated cost for the patient [23]. Prior research of low-income African American women in the United States has shown that mistrust of the medical system, inadequate education about screening, and the presence of barriers (e.g. lack of childcare and transportation) may limit the ability of some individuals to attend screening [24, 25]. Work is needed to determine if similar barriers to screening exist in Ontario, preventing low- and middle-income women from attaining the same degree of screening as their higher-income counterparts.
Notably, while we did observe differences in screening rates with neighbourhood income, when comparing non-screeners to screeners, this effect was limited to women living in an urban setting, with no observed differences in screening behaviour based on neighbourhood income for individuals living in a rural area. When comparing non-screeners to screeners in urban areas, differences in screening behaviour were observed for individuals with low, middle, and high neighbourhood income. Here, the odds of being a non-screener were highest for individuals in the lowest income category living in large urban centres (OR 1.52, 95% CI 1.38, 1.67 compared to high-income individuals). This suggests that screening behaviour differences associated with the effects of income are most persistent for those living in urban environments and not in rural areas. Similar research into the interaction of income and community size on breast screening has been performed in low-income countries [26]. Here, it was found that both urban and rural residing women residing in lower-income neighbourhoods had significantly lower odds of mammography attendance relative to women in higher-income neighbourhoods; however, the effect size for rural residing women was smaller in comparison with urban residing women [26]. Further research into breast cancer screening behaviour should focus on what specific barriers to screening exist for women living in low-income, urban neighbourhoods that do not exist for women residing in low-income rural areas.
The effectiveness of breast cancer screening programmes in reducing the incidence of advanced stage breast cancer has been shown in multiple studies [4, 5, 27,28,29]. Consistent with these findings, the results from this analysis show a clear gradient of an increasing odds of stage II (OR 1.91, 95% CI 1.82, 2.01), III (OR 2.96, 95% CI 2.76, 3.17), and IV (OR 8.96, 95% CI 7.94, 10.12) cancers in non-screeners. A similar pattern was observed for non-adherent screeners, albeit with attenuated effect sizes (Table 4). These results suggest that, while any screening is beneficial, regular breast cancer screening according to OBSP guidelines, is most effective in reducing later stage cancer diagnoses. While performance measures of the OBSP have been analysed [14, 30, 31], to our knowledge, this is the first analysis to look at differences in cancer stage for breast cancer patients who did or did not participate in the OBSP during this time-period. These results highlight the effectiveness of breast screening in Ontario in achieving the goal of reducing the incidence of later stage disease.
Rates of DCIS diagnosis are known to be higher in a screening population [32]. Accordingly, in this study we found non-screeners to be less likely to be diagnosed with DCIS compared to adherent screeners (OR 0.91, 95% CI 0.84, 0.98). It was expected that more cases of DCIS would be found in adherent screeners (11.7%) compared to non-adherent (9.8%) and non-screeners (7.1%).
This study has numerous strengths, including the use of a large population-based cohort of women diagnosed with breast cancer identified through a provincial cancer registry. This allowed for robust comparisons between adherent screeners and non-screeners. The OCR also includes detailed information on tumour characteristics (e.g. stage, ER-status) that can be linked to demographic characteristics of the women within this population.
Limitations of this study include a lack of information on personal income levels, race/ethnicity, and immigration status of women diagnosed with breast cancer in Ontario. The absence of these key demographic variables limited our ability to examine their impact on breast cancer screening behaviours and therefore limited the conclusions we could draw from this current analysis. Further, the study population only included women who were diagnosed with breast cancer, so when examining demographic characteristics associated with breast cancer screening behaviour, these relationships may only exist among women diagnosed with breast cancer. However, screening behaviours were captured through OBSP records and only included screens conducted prior to diagnosis, and all women known to be at high risk of breast cancer (i.e. screened as part of the High Risk OBSP) were excluded from the analysis. Because the coverage of the OCR and the OBSP is province-wide, it is expected that the screening behaviours in this study population reflect those of the broader population. Further evidence of this is seen in the similar screening rates (66.7%) in the study population, as compared to the general population of Ontario in 2018 (66.0%) [3]. Another limitation is that eligible women who exclusively screened outside OBSP would have been classified as non-screeners in this sample since only OBSP screening data were available. However, it would be expected that screening adherent women misclassified as non-screening would attenuate the associations seen with cancer stage at diagnosis.
This research highlights the important differences between women who are screened according to the guidelines of a province-wide, publicly funded screening programme, and those who are screened, but do not meet these guidelines or do not get screened at all. Women who underwent any screening were less likely to have been diagnosed with late-stage breast cancer; however, comparing screen adherent women to non-screening women showed the strongest association. Furthermore, being younger, urban residing, or having a lower neighbourhood income were all associated with a greater likelihood of not undergoing any breast cancer screening. Notably, few women were non-adherent screeners, suggesting that once women initiate screening, most tend to follow the guidelines set out by OBSP. This highlights the need for focused interventions aimed at increasing screening initiation among urban residing, low-income women, to increase screening rates and ensure that more breast cancers are detected before they progress to more advanced and serious stages.
Data availability
The data that support the findings of this study are available from Ontario Health (Cancer Care Ontario), a prescribed entity under Section 45 of the Personal Health Information Protection Act. Data sharing regulations prevent these data from being made available publicly due to the personal health information in the datasets. Data are, however, available from the authors upon reasonable request and with permission of Ontario Health (Cancer Care Ontario).
References
(2021) Canadian Cancer Statistics Advisory Committee in collaboration with the Canadian Cancer Society, Statistics Canada and the Public Health Agency of Canada, Canadian Cancer Statistics 2021. Toronto ON: Canadian Cancer Society
Ontario Breast Screening Program (OBSP). Cancer care Ontario 2017. https://www.cancercareontario.ca/en/cancer-care-ontario/programs/screening-programs/ontario-breast-obsp. Accessed 10 July 2021
Ontario Health (Cancer Care Ontario) (2021) Ontario cancer screening performance report 2020. Toronto: Ontario Health
Khil L, Heidrich J, Wellmann I, Kääb-Sanyal V, Weigel S, Heindel W et al (2020) Incidence of advanced-stage breast cancer in regular participants of a mammography screening program: a prospective register-based study. BMC Cancer 20:174. https://doi.org/10.1186/s12885-020-6646-5
de Munck L, Siesling S, Fracheboud J, den Heeten GJ, Broeders MJM, de Bock GH (2020) Impact of mammographic screening and advanced cancer definition on the percentage of advanced-stage cancers in a steady-state breast screening programme in the Netherlands. Br J Cancer 123:1191–1197. https://doi.org/10.1038/s41416-020-0968-6
Autier P, Héry C, Haukka J, Boniol M, Byrnes G (2009) Advanced breast cancer and breast cancer mortality in randomized controlled trials on mammography screening. J Clin Oncol 27:5919–5923. https://doi.org/10.1200/JCO.2009.22.7041
Doescher MP, Jackson JE (2009) Trends in cervical and breast cancer screening practices among women in rural and urban areas of the United States. J Public Health Manag Pract 15:200–209. https://doi.org/10.1097/PHH.0b013e3181a117da
Aarts MJ, Voogd AC, Duijm LEM, Coebergh JWW, Louwman WJ (2011) Socioeconomic inequalities in attending the mass screening for breast cancer in the south of the Netherlands—associations with stage at diagnosis and survival. Breast Cancer Res Treat 128:517–525. https://doi.org/10.1007/s10549-011-1363-z
Potter M. Phyllodes Tumors - Johns Hopkins Kimmel Cancer Center (n.d.) https://www.hopkinsmedicine.org/kimmel_cancer_center/cancers_we_treat/breast_cancer_program/treatment_and_services/rare_breast_tumors/phyllodes_tumors.html. Accessed 9 Aug 2021
Singletary SE, Connolly JL (2006) Breast cancer staging: working with the sixth edition of the AJCC cancer staging manual. CA A Cancer J Clin 56:37–47. https://doi.org/10.3322/canjclin.56.1.37
MIZ: Detailed definition (n.d.) https://www150.statcan.gc.ca/n1/pub/92-195-x/2011001/other-autre/miz-zim/def-eng.htm. Accessed 13 July 2021
Canadian Institute for Health Information. Measuring health inequalities: a toolkit—area-level equity stratifiers using PCCF and PCCF+ 2018:13.
Austin PC (2009) Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 28:3083–3107. https://doi.org/10.1002/sim.3697
Chiarelli AM, Halapy E, Nadalin V, Shumak R, O’Malley F, Mai V (2006) Performance measures from 10 years of breast screening in the Ontario breast screening program, 1990/91 to 2000. Eur J Cancer Prev 15:34–42. https://doi.org/10.1097/01.cej.0000195713.02567.36
Tatla RK, Paszat LF, Bondy SJ, Chen Z, Chiarelli AM, Mai V (2003) Socioeconomic status & returning for a second screen in the Ontario breast screening program. Breast 12:237–246. https://doi.org/10.1016/S0960-9776(03)00100-0
Orwat J, Caputo N, Key W, De Sa J (2017) Comparing rural and urban cervical and breast cancer screening rates in a privately insured population. Soc Work Public Health 32:311–323. https://doi.org/10.1080/19371918.2017.1289872
Tran L, Tran P (2019) US urban–rural disparities in breast cancer-screening practices at the national, regional, and state level, 2012–2016. Cancer Causes Control 30:1045–1055. https://doi.org/10.1007/s10552-019-01217-8
Leung J, McKenzie S, Martin J, Dobson A, McLaughlin D (2014) Longitudinal patterns of breast cancer screening: mammography, clinical, and breast self-examinations in a rural and urban setting. Women’s Health Issues 24:e139–e146. https://doi.org/10.1016/j.whi.2013.11.005
Stamenić V, Strnad M (2011) Urban-rural differences in a population-based breast cancer screening program in Croatia. Croat Med J 52:76–86. https://doi.org/10.3325/cmj.2011.52.76
Chandak A, Nayar P, Lin G (2019) Rural-urban disparities in access to breast cancer screening: a spatial clustering analysis. J Rural Health 35:229–235. https://doi.org/10.1111/jrh.12308
Katz SJ, Zemencuk JK, Hofer TP (2000) Breast cancer screening in the United States and Canada, 1994: socioeconomic gradients persist. Am J Public Health 90:799–803
Akinyemiju T, Ogunsina K, Sakhuja S, Ogbhodo V, Braithwaite D (2016) Life-course socioeconomic status and breast and cervical cancer screening: analysis of the WHO’s study on global ageing and adult health (SAGE). BMJ Open 6:e012753. https://doi.org/10.1136/bmjopen-2016-012753
Ontario Breast Screening Program. OntarioCa 2014. https://www.ontario.ca/page/ontario-breast-screening-program. Accessed 31 Aug 2021
Garza MA, Luan J, Blinka M, Farabee-Lewis RI, Neuhaus CE, Zabora JR et al (2005) A culturally targeted intervention to promote breast cancer screening among low-income women in East Baltimore. Maryland Cancer Control 12:34–41. https://doi.org/10.1177/1073274805012004S06
Klassen AC, Smith KC, Shariff-Marco S, Juon H-S (2008) A healthy mistrust: how worldview relates to attitudes about breast cancer screening in a cross-sectional survey of low-income women. Int J Equity Health 7:5. https://doi.org/10.1186/1475-9276-7-5
Akinyemiju TF (2012) Socio-economic and health access determinants of breast and cervical cancer screening in low-income countries: analysis of the world health survey. PLoS ONE 7:e48834. https://doi.org/10.1371/journal.pone.0048834
Oluwole SF, Ali AO, Adu A, Blane BP, Barlow B, Oropeza R et al (2003) Impact of a cancer screening program on breast cancer stage at diagnosis in a medically underserved urban community. J Am Coll Surg 196:180–188. https://doi.org/10.1016/S1072-7515(02)01765-9
Tong S, Warner-Smith M, McGill S, Roder D, Currow D, Tong S et al (2020) Effect of mammography screening and sociodemographic factors on stage of female breast cancer at diagnosis in New South Wales. Aust Health Review 44:944–951. https://doi.org/10.1071/AH19124
Hathaway C, Paetsch P, Li Y, Wu J, Asgarian S, Parker A et al (2021) Association of breast cancer screening behaviors with stage at breast cancer diagnosis and potential for additive multi-cancer detection via liquid biopsy screening: a claims-based study. Front Oncol 11:688455. https://doi.org/10.3389/fonc.2021.688455
Chiarelli AM, Blackmore KM, Muradali D, Done SJ, Majpruz V, Weerasinghe A et al (2020) performance measures of magnetic resonance imaging plus mammography in the high risk Ontario breast screening program. JNCI J Natl Cancer Inst 112:136–144. https://doi.org/10.1093/jnci/djz079
Chiarelli AM, Blackmore KM, Mirea L, Done SJ, Majpruz V, Weerasinghe A et al (2020) Annual vs biennial screening: diagnostic accuracy among concurrent cohorts within the Ontario breast screening program. JNCI J Natl Cancer Inst 112:400–409. https://doi.org/10.1093/jnci/djz131
Ernster VL, Barclay J, Kerlikowske K, Grady D, Henderson C (1996) Incidence of and treatment for ductal carcinoma in situ of the breast. JAMA 275:913–918
Acknowledgements
Parts of this material are based on data and information provided by Ontario Health (Cancer Care Ontario) [and includes data received by Ontario Health (Cancer Care Ontario) from the Canadian Institute for Health Information (CIHI)]. The opinions, reviews, views, and conclusions reported in this publication are those of the authors and do not necessarily reflect those of Ontario Health (Cancer Care Ontario) [CIHI]. No endorsement by Ontario Health (Cancer Care Ontario) [CIHI] is intended or should be inferred.
Funding
This study was supported by the Canadian Institutes for Health Research (CIHR) Catalyst Grant: 155465 (GMA).
Author information
Authors and Affiliations
Contributions
GMA and JDB conceived of the design of the study, oversaw analysis, and drafted the manuscript. NG and RAGC conducted the data analysis and drafted the manuscript. RAGC, JA, and AA contributed to study design, data analysis, and drafting of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no financial or non-financial competing interests.
Ethical approval
This study was approved by the Research Ethics Board at the University of Toronto. Data were provided by Ontario Health (Cancer Care Ontario), a prescribed entity under Ontario’s privacy legislation, which is authorized to collect and use personal health information for the purpose of research. Therefore, participant consent was not required.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gold, N., Christensen, R.A.G., Arneja, J. et al. Screening behaviours, demographics, and stage at diagnosis in the publicly funded Ontario Breast Screening Program. Breast Cancer Res Treat 198, 523–533 (2023). https://doi.org/10.1007/s10549-022-06848-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06848-1