Abstract
Purpose
Breast cancer patients with metabolic syndrome (MetS) and its components show worse treatment responses to chemotherapy. Metformin is a widely used antidiabetic drug which also shows potential anticancer effect. This study aims to evaluate the efficacy, safety, and metabolic parameters change of metformin combined with docetaxel, epirubicin, and cyclophosphamide (TEC) in neoadjuvant treatment (NAT) for breast cancer patients with metabolic abnormality.
Methods
Eligible breast cancer patients were randomized to receive six cycles of TEC (docetaxel 75 mg/m2, epirubicin 75 mg/m2, and cyclophosphamide 500 mg/m2, d1, q3w) or TEC with metformin (TECM, TEC with oral metformin 850 mg once daily for the first cycle, then 850 mg twice daily for the following cycles). The primary end point was total pathological complete response (tpCR, ypTis/0N0) rate.
Results
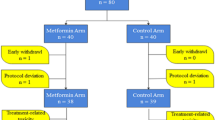
Ninety-two patients were enrolled and randomized from October 2013 to December 2019: 88 patients were available for response and safety assessment. The tpCR rates were 12.5% (5/40) and 14.6% (7/48) in the TEC and TECM groups, respectively (P = 0.777). There was no difference in Ki67 decrease after NAT between two groups (P = 0.456). Toxicity profile were similar between two groups. No grade 3 or higher diarrhea were recorded. Total cholesterol (TC) and high-density lipoprotein cholesterol worsened after NAT in the TEC arm but remained stable in the TECM arm. The absolute increase of TC and low-density lipoprotein cholesterol (LDL-C) was significantly lower in the TECM group compared with the TEC group. After a median follow-up of 40.8 (4.7–70.8) months, no survival difference was observed between TEC and TECM groups (all P > 0.05).
Conclusion
Adding metformin to TEC didn’t increase pCR rate and disease outcome in breast cancer patients with metabolic abnormality. However, additional metformin treatment with chemotherapy would prevent TC and LDL-C increase after NAT.
Trial Registration ClinicalTrials.gov Identifier: NCT01929811.


Similar content being viewed by others
Data availability
The datasets analyzed in the present study are available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Fuchs HE et al (2021) Cancer statistics. CA Cancer J Clin 71(1):7–33. https://doi.org/10.3322/caac.21654
Kaufmann M, von Minckwitz G, Mamounas EP et al (2012) Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol 19(5):1508–1516. https://doi.org/10.1245/s10434-011-2108-2
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172. https://doi.org/10.1016/S0140-6736(13)62422-8
Rastogi P, Anderson SJ, Bear HD et al (2008) Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol 26(5):778–785. https://doi.org/10.1200/JCO.2007.15.0235
Green MC, Buzdar AU, Smith T et al (2005) Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol 23(25):5983–5992. https://doi.org/10.1200/JCO.2005.06.232
Yao X, Hosenpud J, Chitambar CR et al (2012) A phase II study of concurrent docetaxel, epirubicin and cyclophosphamide as a neoadjuvant chemotherapy regimen in patients with locally advanced breast cancer. J Cancer 3:145–151. https://doi.org/10.7150/jca.3980
Chen X, Ye G, Zhang C et al (2013) Superior outcome after neoadjuvant chemotherapy with docetaxel, anthracycline, and cyclophosphamide versus docetaxel plus cyclophosphamide: results from the NATT trial in triple negative or HER2 positive breast cancer. Breast Cancer Res Treat 142(3):549–558. https://doi.org/10.1007/s10549-013-2790-910.1007/s10549-013-2761-1
Bjorge T, Lukanova A, Jonsson H et al (2010) Metabolic syndrome and breast cancer in the me-can (metabolic syndrome and cancer) project. Cancer Epidemiol Biomarkers Prev 19(7):1737–1745. https://doi.org/10.1158/1055-9965.EPI-10-0230
Bhandari R, Kelley GA, Hartley TA et al (2014) Metabolic syndrome is associated with increased breast cancer risk: a systematic review with meta-analysis. Int J Breast Cancer 2014:189384. https://doi.org/10.1155/2014/189384
Stebbing J, Sharma A, North B et al (2012) A metabolic phenotyping approach to understanding relationships between metabolic syndrome and breast tumour responses to chemotherapy. Ann Oncol 23(4):860–866. https://doi.org/10.1093/annonc/mdr347
Decensi A, Puntoni M, Goodwin P et al (2010) Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 3(11):1451–1461. https://doi.org/10.1158/1940-6207.CAPR-10-0157
Dankner R, Agay N, Olmer L et al (2019) Metformin treatment and cancer risk: cox regression analysis, with time-dependent covariates, of 320,000 persons with incident diabetes mellitus. Am J Epidemiol 188(10):1794–1800. https://doi.org/10.1093/aje/kwz157
Tang GH, Satkunam M, Pond GR et al (2018) Association of metformin with breast cancer incidence and mortality in patients with type II diabetes: a grade-assessed systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 27(6):627–635. https://doi.org/10.1158/1055-9965.EPI-17-0936
Jiralerspong S, Palla SL, Giordano SH et al (2009) Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol 27(20):3297–3302. https://doi.org/10.1200/JCO.2009.19.6410
Martin-Castillo B, Pernas S, Dorca J et al (2018) A phase 2 trial of neoadjuvant metformin in combination with trastuzumab and chemotherapy in women with early HER2-positive breast cancer: the METTEN study. Oncotarget 9(86):35687–35704. https://doi.org/10.18632/oncotarget.26286
Barakat HE, Hussein RRS, Elberry AA et al (2022) The impact of metformin use on the outcomes of locally advanced breast cancer patients receiving neoadjuvant chemotherapy: an open-labelled randomized controlled trial. Sci Rep 12(1):7656. https://doi.org/10.1038/s41598-022-11138-3
Goodwin PJ, Chen BE, Gelmon KA et al (2022) Effect of metformin vs placebo on invasive disease-free survival in patients with breast cancer: the MA.32 randomized clinical trial. JAMA 327(20):1963–1973. https://doi.org/10.1001/jama.2022.6147
Liu J, Grundy SM, Wang W et al (2006) Ethnic-specific criteria for the metabolic syndrome: evidence from China. Diabetes Care 29(6):1414–1416. https://doi.org/10.2337/dc06-0481
Group., C.D.S.M.S.R (2004) Metabolic syndrome: suggestion from Chinese Diabetes Society. Chin J Diabetes 12(3):156–161
De A, Kuppusamy G (2020) Metformin in breast cancer: preclinical and clinical evidence. Curr Probl Cancer 44(1):100488. https://doi.org/10.1016/j.currproblcancer.2019.06.003
GoDarts, U.D.P.S. Group, C. Wellcome Trust Case Control et al (2011) Common variants near ATM are associated with glycemic response to metformin in type 2 diabetes. Nat Genet 43(2):117–20. https://doi.org/10.1038/ng.735
Cuyas E, Buxo M, Ferri Iglesias MJ et al (2019) The C Allele of ATM rs11212617 associates with higher pathological complete remission rate in breast cancer patients treated with neoadjuvant metformin. Front Oncol. https://doi.org/10.3389/fonc.2019.00193
Niraula S, Dowling RJ, Ennis M et al (2012) Metformin in early breast cancer: a prospective window of opportunity neoadjuvant study. Breast Cancer Res Treat 135(3):821–830. https://doi.org/10.1007/s10549-012-2223-1
Hadad S, Iwamoto T, Jordan L et al (2011) Evidence for biological effects of metformin in operable breast cancer: a pre-operative, window-of-opportunity, randomized trial. Breast Cancer Res Treat 128(3):783–794. https://doi.org/10.1007/s10549-011-1612-1
Bonanni B, Puntoni M, Cazzaniga M et al (2012) Dual effect of metformin on breast cancer proliferation in a randomized presurgical trial. J Clin Oncol 30(21):2593–2600. https://doi.org/10.1200/JCO.2011.39.3769
Kalinsky K, Crew KD, Refice S et al (2014) Presurgical trial of metformin in overweight and obese patients with newly diagnosed breast cancer. Cancer Invest 32(4):150–157. https://doi.org/10.3109/07357907.2014.889706
Pistelli M, Merloni F, Crocetti S et al (2021) Prognostic impact of Ki-67 change in locally advanced and early breast cancer after neoadjuvant chemotherapy: a single institution experience. J Oncol 2021:5548252. https://doi.org/10.1155/2021/5548252
Luo N, Ji Y, Huang X et al (2019) Changes in apparent diffusion coefficient as surrogate marker for changes in ki-67 index due to neoadjuvant chemotherapy in patients with invasive breast cancer. Acad Radiol 26(10):1352–1357. https://doi.org/10.1016/j.acra.2019.01.007
Goodwin PJ, Parulekar WR, Gelmon KA et al (2015) Effect of metformin vs placebo on and metabolic factors in NCIC CTG MA.32. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djv006
Wang Q, Ma X, Long J et al (2022) Metformin and survival of women with breast cancer: a meta-analysis of randomized controlled trials. J Clin Pharm Ther 47(3):263–269. https://doi.org/10.1111/jcpt.13500
Kim BH, Cho MJ, Kwon J (2021) Potential intrinsic subtype dependence on the association between metformin use and survival in surgically resected breast cancer: a Korean national population-based study. Int J Clin Oncol 26(11):2004–2016. https://doi.org/10.1007/s10147-021-02005-8
Chae YK, Arya A, Malecek MK et al (2016) Repurposing metformin for cancer treatment: current clinical studies. Oncotarget 7(26):40767–40780. https://doi.org/10.18632/oncotarget.8194
Acknowledgements
The authors are grateful to the patients, their families, and caregivers for participating in NeoMET trial. The authors also thank the study investigators and site staff for their participation.
Funding
This study was funded by the National Natural Science Foundation of China (82072937, 82072897); Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (20172007); Shanghai Jiao Tong University Yi Gong Jiao Cha Funding (YG2019QNA30); and Ruijin Hospital, Shanghai Jiao Tong University School of Medicine- “Guangci Excellent Youth Training Program” (GCQN-2019-B07). All these financial sponsors had no role in the study design, data collection, analysis, or interpretation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
The present study was reviewed and approved by independent ethical committees of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine (2013-32a/2013–7-31). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, J., Tong, Y., Hong, J. et al. Neoadjuvant docetaxel, epirubicin, and cyclophosphamide with or without metformin in breast cancer patients with metabolic abnormality: results from the randomized Phase II NeoMET trial. Breast Cancer Res Treat 197, 525–533 (2023). https://doi.org/10.1007/s10549-022-06821-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06821-y