Abstract
Purpose
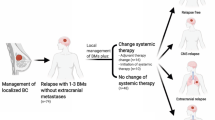
Current systemic therapy guidelines for patients with HER2 + breast cancer brain metastases (BCBrM) diverge based on the status of extracranial disease (ECD). An in-depth understanding of the impact of ECD on outcomes in HER2 + BCBrM has never been performed. Our study explores the implications of ECD status on intracranial progression-free survival (iPFS) and overall survival (OS) after first incidence of HER2 + BCBrM and radiation.
Methods
A retrospective analysis was performed of 151 patients diagnosed with initial HER2 + BCBrM who received radiation therapy to the central nervous system (CNS) at Duke between 2008 and 2021. The primary endpoint was iPFS defined as the time from first CNS radiation treatment to intracranial progression or death. OS was defined as the time from first CNS radiation or first metastatic disease to death. Systemic staging scans within 30 days of initial BCBrM defined ECD status as progressive, stable/responding or none (isolated brain relapse).
Results
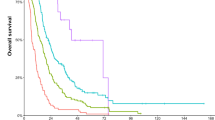
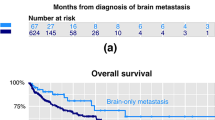
In this cohort, > 70% of patients had controlled ECD with either isolated brain relapse (27%) or stable/responding ECD (44%). OS from initial metastatic disease to death was markedly worse for patients with isolated intracranial relapse (median = 28.4 m) compared to those with progressive or stable/responding ECD (48.8 m and 71.5 m, respectively, p = 0.0028). OS from first CNS radiation to death was significantly worse for patients with progressive ECD (16.9 m) versus stable/responding (36.6 m) or isolated intracranial relapse (28.4 m, p = 0.007). iPFS did not differ statistically based on ECD status. Receipt of systemic therapy after first BCBrM significantly improved iPFS (HR 0.45, 95% CI: 0.25–0.81, p = 0.008) and OS (HR: 0.43 (95% CI: 0.23–0.81); p = 0.001).
Conclusion
OS in patients with HER2 + isolated BCBrM was inferior to those with concurrent progressive or stable/responding ECD. Studies investigating initiation of brain-penetrable HER2-targeted therapies earlier in the disease course of isolated HER2 + intracranial relapse patients are warranted.




Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are not publicly available as they contain identifying information but available from the corresponding author on reasonable request.
References
Bailleux C, Eberst L, Bachelot T (2021) Treatment strategies for breast cancer brain metastases. Br J Cancer 124(1):142–155
Arslan UY et al (2011) Breast cancer subtypes and outcomes of central nervous system metastases. Breast 20(6):562–567
Rostami R et al (2016) Brain metastasis in breast cancer: a comprehensive literature review. J Neurooncol 127(3):407–414
Vogelbaum MA et al (2022) Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J Clin Oncol 40(5):492–516
Sperduto PW et al (2013) The effect of tumor subtype on the time from primary diagnosis to development of brain metastases and survival in patients with breast cancer. J Neurooncol 112(3):467–472
Leone JP, Lin NU (2019) Systemic therapy of central nervous system metastases of breast cancer. Curr Oncol Rep 21(6):49
Darlix A et al (2019) Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of central nervous system metastases in a large multicentre real-life cohort. Br J Cancer 121(12):991–1000
Niwińska A (2016) Brain metastases as site of first and isolated recurrence of breast cancer: the role of systemic therapy after local treatment. Clin Exp Metastasis 33(7):677–685
Lin NU et al (2020) Intracranial efficacy and survival With tucatinib plus trastuzumab and capecitabine for previously treated her2-positive breast cancer with brain metastases in the HER2CLIMB Trial. J Clin Oncol 38(23):2610–2619
Ramakrishna N et al (2018) Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: ASCO clinical practice guideline update. J Clin Oncol 36(27):2804–2807
Ramakrishna N et al (2022) Management of advanced human epidermal growth factor receptor 2–positive breast cancer and brain metastases: ASCO guideline update. J Clin Oncol. https://doi.org/10.1200/OP.22.00364
Exman P, Tolaney SM (2021) HER2-positive metastatic breast cancer: a comprehensive review. Clin Adv Hematol Oncol 19(1):40–50
Zhang Q et al (2016) Survival benefit of anti-HER2 therapy after whole-brain radiotherapy in HER2-positive breast cancer patients with brain metastasis. Breast Cancer 23(5):732–739
Hackshaw MD et al (2021) Prognostic factors of brain metastasis and survival among HER2-positive metastatic breast cancer patients: a systematic literature review. BMC Cancer 21(1):967
PART 46 - PROTECTION OF HUMAN SUBJECTS. Code of Federal Regulations, 2018.
Wolff AC et al (2018) Human epidermal growth factor receptor 2 testing in breast cancer: american society of clinical oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol 36(20):2105–2122
Lin NU et al (2015) Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 16(6):e270–e278
von Minckwitz G et al (2018) Trastuzumab Emtansine for residual Invasive HER2-positive breast cancer. N Engl J Med 380(7):617–628
Untch M et al (2019) Peripheral neuropathy (PN), thrombocytopenia (TCP) and central nervous system (CNS) recurrence: an update of the phase III KATHERINE trial of post-neoadjuvant trastuzumab emtansine (T-DM1) or trastuzumab (H) in patients (pts) with residual invasive HER2-positive breast cancer (BC). Ann Oncol 30:851–934
Swain SM et al (2015) Pertuzumab, Trastuzumab, and Docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 372(8):724–734
Stemmler H-J et al (2007) Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood–brain barrier. Anticancer Drugs 18(1):23–28
Funding
Funding for this project was provided to Laura Noteware by the Stead Scholarship Program of the Duke University Department of Medicine. Scott Floyd is supported by grants from the NIH (NIH 5R01-NS100866-05, NIH U01TR0033715-01, and NIH 5R38-CA245204-02) and the American Cancer Society (13394-RSG-19–030-01-DMC). Additional research funding from PUMA, Lilly, Merck, Seattle Genetics, Nektar, Tesaro, G1-Therapeutics, ZION, Novartis, and Pfizer were provided to Carey Anders. Sarah Sammons receives institutional funding from Astra Zeneca, Abbvie, Bristol Myers Squibb, Eli Lilly, SEAGEN, and Sermonix. The authors declare that no additional funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Carey Anders has been a compensated consultant for Genentech, Eisai, IPSEN, Seattle Genetics, Astra Zeneca, Novartis, Immunomedics, Elucida, and Athenex. Dr. Anders also receives royalties from UpToDate, Jones and Bartlett. Sarah Sammons is a compensated consultant at Foundation Medicine, Astra Zeneca, Daichii Sankyo, Eli Lilly, Pfizer, Sermonix, and Novartis. The additional authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Noteware, L., Broadwater, G., Dalal, N. et al. Brain metastasis as the first and only metastatic relapse site portends worse survival in patients with advanced HER2 + breast cancer. Breast Cancer Res Treat 197, 425–434 (2023). https://doi.org/10.1007/s10549-022-06799-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06799-7