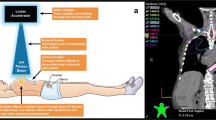
Abstract
Purpose
Ovarian stimulation for oocyte and embryo cryopreservation is the standard of care for fertility preservation in young breast cancer patients before gonadotoxic chemotherapy. The procedure should be started as soon as possible to avoid delay of treatment; thus, it is often performed concomitantly with tumor staging assessments. However, questions remain regarding the potential negative impact on oocyte quality that may occur due to exposure to scattered ionizing radiation from imaging techniques when staging assessment is conducted at the same time as ovarian stimulation.
Methods
We conducted a retrospective study on all breast cancer patients who performed ovarian stimulation for fertility preservation at our center between November 2012 and May 2020.
Results
Gynecologic and oncological characteristics were similar between patients exposed (n = 14) or not (n = 60) to ionizing radiation. Exposed patients started the ovarian stimulation sooner after diagnosis than non-exposed patients (11.5 vs 28 days, respectively, P < 0.01). Cycle parameters, including the median number of oocytes collected (10.5 vs 7, P = 0.16), maturation rates (92.5% vs 85.7%, P = 0.54), and fertilization rates (62.2% vs 65.4%, P = 0.70), were similar between groups.
Conclusion
This study shows that scattered ionizing radiation due to staging assessment appears to be safe without compromising follicular growth and maturation. Larger studies on fertility and obstetrical outcomes are needed to confirm these preliminary data.
Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Fidler MM, Gupta S, Soerjomataram I et al (2017) Cancer incidence and mortality among young adults aged 20–39 years worldwide in 2012: a population-based study. Lancet Oncol 18:1579–1589. https://doi.org/10.1016/S1470-2045(17)30677-0
Heer E, Harper A, Escandor N et al (2020) Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health 8:e1027–e1037. https://doi.org/10.1016/S2214-109X(20)30215-1
Copson E, Maishman T, Gerty S et al (2014) Ethnicity and outcome of young breast cancer patients in the United Kingdom: the POSH study. Br J Cancer 110:230–241. https://doi.org/10.1038/bjc.2013.650
Anderson RA, Amant F, Braat D et al (2020) ESHRE guideline: female fertility preservation. Hum Reprod Open. https://doi.org/10.1093/hropen/hoaa052
Lambertini M, Peccatori FA, Demeestere I et al (2020) Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. https://doi.org/10.1016/j.annonc.2020.09.006
Paluch-Shimon S, Cardoso F, Partridge AH et al (2020) ESO-ESMO 4th international consensus guidelines for breast cancer in young women (BCY4). Ann Oncol 31:674–696. https://doi.org/10.1016/j.annonc.2020.03.284
Oktay K, Harvey BE, Partridge AH et al (2018) Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol 36:1994–2001. https://doi.org/10.1200/JCO.2018.78.1914
Practice Committee of the American Society for Reproductive Medicine. Electronic address AAO (2019) Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril 112:1022–1033. https://doi.org/10.1016/j.fertnstert.2019.09.013
Bonardi B, Massarotti C, Bruzzone M et al (2020) Efficacy and safety of controlled ovarian stimulation with or without letrozole co-administration for fertility preservation: a systematic review and meta-analysis. Front Oncol 10:574669. https://doi.org/10.3389/fonc.2020.574669
Cardoso F, Kyriakides S, Ohno S et al (2019) Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 30:1674. https://doi.org/10.1093/annonc/mdz189
Chien AJ, Chambers J, McAuley F et al (2017) Fertility preservation with ovarian stimulation and time to treatment in women with stage II–III breast cancer receiving neoadjuvant therapy. Breast Cancer Res Treat 165:151–159. https://doi.org/10.1007/s10549-017-4288-3
Kirk M, Lyon MF (1982) Induction of congenital anomalies in offspring of female mice exposed to varying doses of X-rays. Mutat Res 106:73–83. https://doi.org/10.1016/0027-5107(82)90191-9
Meirow D, Epstein M, Lewis H et al (2001) Administration of cyclophosphamide at different stages of follicular maturation in mice: effects on reproductive performance and fetal malformations. Hum Reprod (Oxford, England) 16:632–637. https://doi.org/10.1093/humrep/16.4.632
Stringer JM, Winship A, Zerafa N et al (2020) Oocytes can efficiently repair DNA double-strand breaks to restore genetic integrity and protect offspring health. Proc Natl Acad Sci USA 117:11513–11522. https://doi.org/10.1073/pnas.2001124117
Marangos P, Carroll J (2012) Oocytes progress beyond prophase in the presence of DNA damage. Curr Biol 22:989–994. https://doi.org/10.1016/j.cub.2012.03.063
Gougeon A (1996) Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev 17:121–155
Goldrat O, Gervy C, Englert Y et al (2015) Progesterone levels in letrozole associated controlled ovarian stimulation for fertility preservation in breast cancer patients. Hum Reprod (Oxford, England) 30:2184–2189. https://doi.org/10.1093/humrep/dev155
Radiology ACo (2018) ACR–SPR practice parameter for imaging pregnant or potentially pregnant adolescents and women with ionizing radiation. https://www.acr.org/Clinical-Resources/Radiology-Safety/Radiation-Safety
McCollough CH, Schueler BA, Atwell TD et al (2007) Radiation exposure and pregnancy: when should we be concerned? Radiographics 27:909–917; discussion 917–908. https://doi.org/10.1148/rg.274065149
Borrego-Soto G, Ortiz-Lopez R, Rojas-Martinez A (2015) Ionizing radiation-induced DNA injury and damage detection in patients with breast cancer. Genet Mol Biol 38:420–432. https://doi.org/10.1590/S1415-475738420150019
Wallace WH, Thomson AB, Saran F et al (2005) Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys 62:738–744. https://doi.org/10.1016/j.ijrobp.2004.11.038
Jacquet P, Adriaens I, Buset J et al (2005) Cytogenetic studies in mouse oocytes irradiated in vitro at different stages of maturation, by use of an early preantral follicle culture system. Mutat Res 583:168–177. https://doi.org/10.1016/j.mrgentox.2005.03.008
Yuen WS, Merriman JA, O’Bryan MK et al (2012) DNA double strand breaks but not interstrand crosslinks prevent progress through meiosis in fully grown mouse oocytes. PLoS ONE 7:e43875. https://doi.org/10.1371/journal.pone.0043875
Collins JK, Lane SIR, Merriman JA et al (2015) DNA damage induces a meiotic arrest in mouse oocytes mediated by the spindle assembly checkpoint. Nat Commun 6:8553. https://doi.org/10.1038/ncomms9553
Suh EK, Yang A, Kettenbach A et al (2006) p63 protects the female germ line during meiotic arrest. Nature 444:624–628. https://doi.org/10.1038/nature05337
Acknowledgements
The authors would like to acknowledge the contribution of a medical writer, Sandy Field, PhD, for English language editing of this manuscript.
Funding
Margherita Condorelli and Isabelle Demeestere acknowledge Télévie-FNRS and Fonds Erasme for their financial support. This work was supported by Télévie-FNRS (Grant Number: 7.6508.20) and Fonds Erasme (Grant Number: not applicable). Matteo Lambertini acknowledges the support from the Italian Association for Cancer Research (“Associazione Italiana per la Ricerca sul Cancro,” AIRC; MFAG 2020 ID 24698) and the Italian Ministry of Health (5 x 1000 funds 2017).
Author information
Authors and Affiliations
Contributions
MC, ML, and ID conceived and designed the study, OG, MS, AD, and MC acquired the data. JR, ML, MS, and MC analyzed the data. MC and ID wrote the manuscript and all other authors revised it critically and finally approved it.
Corresponding author
Ethics declarations
Conflict of interest
Matteo Lambertini acted as a consultant for Roche, Lilly, AstraZeneca, and Novartis and has received honoraria from Sandoz, Roche, Lilly, Pfizer, Novartis, and Takeda, outside the submitted work. Anne Delbaere received grants from Ferring Pharmaceuticals and consultancy or lecture fees from Merck, Gedeon-Richter Ferring Pharmaceuticals, and OVVI Diagnostics, outside the submitted work. Isabelle Demeestere received research grants from Ferring and Roche, consultancy or lecture fees from Roche, Novartis and support for attending meetings from Ferring and Theramex, outside the submitted work. The remaining authors have no conflicts of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the ethics committee of CUB-Hôpital Erasme (P2020.328).
Informed consent
The need for obtaining informed consent was waived by the Hôpital Erasme ethical committee, given that this study was retrospective and non-interventional. Patients have the right to refuse to participate in any clinical trial by informing the hospital which keeps a record of their choice. We hereby confirm that we took into account patient preference and consequently excluded all patients that refused their participation in clinical trials.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file4 (JPG 113 kb)
Figure S1: Correlation between radiation doses and total number of oocytes collected (pink) and total number of mature oocytes (blue) in the exposure group.
Rights and permissions
About this article
Cite this article
Condorelli, M., Sens, M., Goldrat, O. et al. A retrospective study evaluating the impact of scattering radiation from imaging procedures on oocyte quality during ovarian stimulation for fertility preservation in young breast cancer patients. Breast Cancer Res Treat 192, 123–130 (2022). https://doi.org/10.1007/s10549-021-06489-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06489-w