Abstract
Background
Clinical and genomic data from patients with early-stage breast cancer suggest more aggressive disease in premenopausal women. However, the association between age, disease course, and molecular profile from liquid biopsy in metastatic breast cancer (MBC) is not well characterized.
Methods
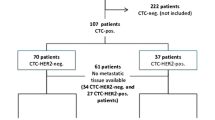
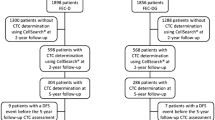
Patients were classified as premenopausal (< 45 years), perimenopausal (45–55 years), or postmenopausal (> 55 years). Cohort 1 consisted of patients with MBC who consented for prospective serial evaluation of circulating tumor cells (CTCs) using CellSearch™. Cohort 2 included patients who, as part of routine care, had circulating tumor DNA (ctDNA) sequenced by the Guardant360™ assay. Clinicopathologic data were collected from retrospective review to compare disease features between premenopausal and postmenopausal women.
Results
Premenopausal women represented 26% of 138 patients in Cohort 1 and 21% of 253 patients in Cohort 2. In Cohort 1, younger patients had a shorter time to metastases and a higher prevalence of lung and brain metastases. Overall, there were similar rates of ≥ 5 CTCs/7.5 mL, HER2 + CTC expression, and CTC clusters between pre- and postmenopausal women. However, for those with triple negative breast cancer, premenopausal women had a higher proportion of ≥ 5 CTCs/7.5 mL. In Cohort 2, premenopausal women had a higher incidence of FGFR1 (OR 2.75, p = 0.022) and CCND2 (OR 6.91, p = 0.024) alterations. There was no difference in the ctDNA mutant allele frequency or the number of detected alterations between these age groups.
Conclusions
Our data reveal that premenopausal women diagnosed with MBC have unique clinical, pathologic, and molecular features when compared to their postmenopausal counterparts. Our results highlight FGFR1 inhibitors as potential therapeutics of particular interest among premenopausal women.



Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due ongoing data collection but are available from the corresponding author on reasonable request.
References
Fidler MM et al (2017) Cancer incidence and mortality among young adults aged 20–39 years worldwide in 2012: a population-based study. Lancet Oncol 18(12):1579–1589
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70(1):7–30
Liao S et al (2015) The molecular landscape of premenopausal breast cancer. Breast Cancer Res 17:104
Fu J et al (2018) How young is too young in breast cancer?-Young breast cancer is not a unique biological subtype. Clin Breast Cancer 18(1):e25–e39
Keegan TH et al (2012) Occurrence of breast cancer subtypes in adolescent and young adult women. Breast Cancer Res 14(2):R55
O’Brien KM et al (2015) Risk factors for young-onset invasive and in situ breast cancer. Cancer Causes Control 26(12):1771–1778
Im S-A et al (2019) Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med 381(4):307–316
Cristofanilli M et al (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351(8):781–791
Hrebien S et al (2019) Early ctDNA dynamics as a surrogate for progression-free survival in advanced breast cancer in the BEECH trial. Ann Oncol 30(6):945–952
Bidard F-C et al (2020) Efficacy of circulating tumor cell count-driven vs clinician-driven first-line therapy choice in hormone receptor-positive, ERBB2-negative metastatic breast cancer: the STIC CTC randomized clinical trial. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2020.5660
Hayashi N et al (2012) Prognostic value of HER2-positive circulating tumor cells in patients with metastatic breast cancer. Int J Clin Oncol 17(2):96–104
Amintas S et al (2020) Circulating tumor cell clusters: united we stand divided we fall. Int J Mol Sci 21(7):2653
Shah AN et al (2019) Abstract P3-01-19: HER2-positive circulating tumor cells (CTCs) in advanced breast cancer (BC): a feature independent of BC subtype. Cancer Res 79(4 Supplement):P3-01–19
Higgins MJ et al (2012) Detection of tumor PIK3CA status in metastatic breast cancer using peripheral blood. Clin Cancer Res 18(12):3462–3469
Anders CK et al (2008) Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 26(20):3324–3330
Kolečková M et al (2017) Age-associated prognostic and predictive biomarkers in patients with breast cancer. Oncol Lett 13(6):4201–4207
Gnerlich JL et al (2009) Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg 208(3):341–347
Azim HA Jr et al (2015) Genomic aberrations in young and elderly breast cancer patients. BMC Med 13:266–266
Cristofanilli M et al (2019) The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): international expert consensus paper. Crit Rev Oncol Hematol 134:39–45
Gao J et al (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6(269):pl1
Tolaney SM et al (2019) Randomized phase II study of eribulin mesylate (E) with or without pembrolizumab (P) for hormone receptor-positive (HR +) metastatic breast cancer (MBC). J Clin Oncol 37(15_suppl):1004
Razavi P et al (2018) The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell 34(3):427-438.e6
Turner N et al (2020) Abstract GS3-06: results from the plasmaMATCH trial: a multiple parallel cohort, multi-centre clinical trial of circulating tumour DNA testing to direct targeted therapies in patients with advanced breast cancer (CRUK/15/010). Cancer Res 80(4 Supplement):GS3-06
Shah AN et al (2020) Hormone receptor-positive/human epidermal growth receptor 2-negative metastatic breast cancer in young women: emerging data in the era of molecularly targeted agents. Oncologist 25(6):e900–e908
Bardia A et al (2019) Abstract PD2-08: Ribociclib with endocrine therapy for premenopausal patients with hormone receptor-positive, HER2-negative advanced breast cancer: biomarker analyses from the phase III randomized MONALEESA-7 trial. Cancer Res 79(4 Supplement):PD2-08
Leary B et al (2018) The genetic landscape and clonal evolution of breast cancer resistance to Palbociclib plus Fulvestrant in the PALOMA-3 trial. Cancer Discov 8(11):1390
Dawood S et al (2008) Circulating tumor cells in metastatic breast cancer. Cancer 113(9):2422–2430
Rack B et al (2014) Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst 106(5):dju066
Turner N et al (2010) FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res 70(5):2085–2094
André F et al (2014) Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: a multicentre, prospective trial (SAFIR01/UNICANCER). Lancet Oncol 15(3):267–274
Perez-Garcia J et al (2018) Targeting FGFR pathway in breast cancer. Breast 37:126–133
Formisano L et al (2019) Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat Commun 10(1):1373
Yamamoto-Ibusuki M, Arnedos M, André F (2015) Targeted therapies for ER+/HER2− metastatic breast cancer. BMC Med 13(1):137
Wang S, Ding Z (2017) Fibroblast growth factor receptors in breast cancer. Tumor Biol 39(5):1010428317698370
Turner NC et al (2020) Abstract OT2-07-01: a phase 2 study of futibatinib (TAS-120) in metastatic breast cancers harboring fibroblast growth factor receptor (FGFR) amplifications (FOENIX-MBC2). Cancer Res 80(4 Supplement):OT2-07–01
Boyle P (2012) Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol 23(Suppl 6):vi7–vi12
Funding
Lynn Sage Research Foundation.
Author information
Authors and Affiliations
Contributions
ANS, KC, LG, and MC involved in concept/design of work; ANS, KC, LG, AAD, QZ, and SJ collected the data; ANS, KC, and LG performed data analysis/interpretation; ANS and KC wrote the manuscript; and all involved in critical revision of the article and final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
Ami N. Shah: Honorarium—Taiho, Daiichi-Sankyo, Pfizer; Advisory Board—d AbbVie; Lorenzo Gerratana: Advisory board—Lilly; Amir Behdad: Fees for Non-CME Services Received Directly from Commercial Interest or their Agents (e.g., speakers' bureaus); Author; Pfizer, Bayer, Foundation Medicine; Massimo Cristofanilli: Advisory Board—G1 Therapeutics, Cytodyn, Sermonix, LIlly, Foundation Medicine. Fees for Non-CME Services Received Directly from Commercial Interest or their Agents—Pfizer. Contracted Research—G1 Therapeutics. Others: none.
Ethical approval
This protocol was approved by the institutional review board at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University (STU00203283 and STU00214133).
Consent to participate
Patients provided written consent to participate in part 1.
Informed consent
Requirement for informed consent was waived for part 2 for this retrospective review of de-identified patient data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shah, A.N., Carroll, K.J., Gerratana, L. et al. Circulating tumor cells, circulating tumor DNA, and disease characteristics in young women with metastatic breast cancer. Breast Cancer Res Treat 187, 397–405 (2021). https://doi.org/10.1007/s10549-021-06236-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06236-1