Abstract
Purpose
Research suggested that bone is the specific target organ for breast cancer metastasis. The related tumor causes significant morbidity due to a reduction in quality of life and physical function. Increased osteoclast function is implicated in the bone microenvironment during the outgrowth of breast cancer. In the present experimental study, we examined the potential bone-protective effect of baicalin osteotropic breast Cancer and explored the possible mechanism of action.
Methods
In vitro cell viability effect of baicalin was assessed on the breast cancer cell lines (MDA-MB-231 and MCF-7). We also estimated the in vitro osteoclast and bone resorption. Further, baicalin-regulated osteoblastogenesis and osteoclastogenesis were also estimated in vitro. Finally, the role of the baicalin in the expansion of osteolytic bone disease was scrutinized in a breast cancer bone metastases model.
Results
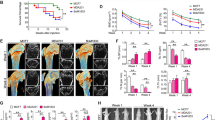
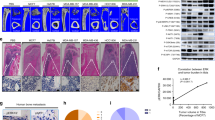
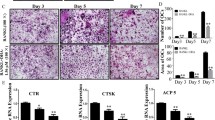
Baicalin significantly (p < 0.001) downregulated the viability of murine and human cancer cell lines and diminished the osteoclastogenesis of osteoclast progenitors via estimation with the help of qRT-PCR. Baicalin showed the downregulation in the mRNA expression of OCN and ALP. Baicalin reduced the TRAP-positive cells in the presence of RANKL. Baicalin considerably upregulated the cytochrome c secretion into the cytoplasm. Baicalin markedly increased the DNA fragmentation, caspase-3, caspase-8, and caspase-9. Baicalin significantly (p < 0.001) reduced the metastatic growth of MDA-MB-231 cells,preserving the bone mass in a bone metastasis model.
Conclusion
Collectively, we can conclude that these results highlight the bone-protective effect of baicalin, which also highlighted the anti-tumor effect; further research is needed into the likely effects on bone health in the bone metastases and osteoporosis populations, such as post-menopausal women with breast cancer.








Similar content being viewed by others
References
Coleman R, Body JJ, Aapro M et al (2014) Bone health in cancer patients: ESMO clinical practice guidelines. Ann Oncol 25:124–137. https://doi.org/10.1093/annonc/mdu103
Sunita P, Pattanayak SP (2011) Phytoestrogens in postmenopausal indications: a theoretical perspective. Pharmacogn Rev 5:41
Nordin ML, Abdul Kadir A, Zakaria ZA et al (2018) In vitro investigation of cytotoxic and antioxidative activities of Ardisia crispa against breast cancer cell lines, MCF-7 and MDA-MB-231. BMC Complement Altern Med. https://doi.org/10.1186/s12906-018-2153-5
Dethlefsen C, Højfeldt G, Hojman P (2013) The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res Treat 138:657–664
Tai KH, Foroudi F (2013) Management of bone metastases. Prostate cancer: a comprehensive perspective. Springer, London
Christakos S, Hewison M, Gardner DG et al (2013) Vitamin D: beyond bone. Ann N Y Acad Sci. https://doi.org/10.1111/nyas.12129
Kang Y, Siegel PM, Shu W et al (2003) A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. https://doi.org/10.1016/S1535-6108(03)00132-6
Marcom PK (2017) Breast cancer. Genomic and precision medicine primary care, 3rded edn. Elsevier, Amsterdam
Early Breast Cancer Trialists Collaborative Group (EBCTCG) (1998) Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet (London, England). https://doi.org/10.1016/S0140-6736(97)11423-4
Karnoub AE, Dash AB, Vo AP et al (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. https://doi.org/10.1038/nature06188
Fernández Vallone VB, Hofer EL, Choi H et al (2013) Behaviour of mesenchymal stem cells from bone marrow of untreated advanced breast and lung cancer patients without bone osteolytic metastasis. Clin Exp Metastasis. https://doi.org/10.1007/s10585-012-9539-4
Marino S, de Ridder D, Bishop RT et al (2019) Paradoxical effects of JZL184, an inhibitor of monoacylglycerol lipase, on bone remodelling in healthy and cancer-bearing mice. EBioMedicine. https://doi.org/10.1016/j.ebiom.2019.05.048
Brufsky AM (2008) Bone health issues in women with early-stage breast cancer receiving aromatase inhibitors. Curr Oncol Rep 10:18–26
Yin JJ, Selander K, Chirgwin JM et al (1999) TGF-β signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest 103:197–206. https://doi.org/10.1172/JCI3523
Roodman GD (2001) Biology of osteoclast activation in cancer. J Clin Oncol. https://doi.org/10.1200/JCO.2001.19.15.3562
Mundy GR, Yoneda T, Hiraga T (2001) Preclinical studies with zoledronic acid and other bisphosphonates: impact on the bone microenvironment. Semin Oncol. https://doi.org/10.1053/sonc.2001.24158
Pavlakis N, Schmidt RL, Stockler MR (2005) Bisphosphonates for breast cancer. Cochrane database of systematic reviews. Wiley, Chichester
Perez EA (2006) The balance between risks and benefits: long-term use of aromatase inhibitors. Eur J Cancer. https://doi.org/10.1016/j.ejcsup.2006.06.003
Marx RE, Sawatari Y, Fortin M, Broumand V (2005) Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg 63:1567–1575
You L, Temiyasathit S, Lee P et al (2008) Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone. https://doi.org/10.1016/j.bone.2007.09.047
World Health Organisation (2017) Breast cancer: prevention and control. World Health Organisation, Geneva
Hwang JM, Tseng TH, Tsai YY et al (2005) Protective effects of baicalein on tert-butyl hydroperoxide-induced hepatic toxicity in rat hepatocytes. J Biomed Sci 12:389–397. https://doi.org/10.1007/s11373-005-1572-8
Yao J, Cao X, Zhang R et al (2016) Protective effect of baicalin against experimental colitis via suppression of oxidant stress and apoptosis. Pharmacogn Mag 12:225–234. https://doi.org/10.4103/0973-1296.186342
Park BK, Zhang H, Zeng Q et al (2007) NF-κB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat Med. https://doi.org/10.1038/nm1519
Tian B, Jiang T, Shao Z et al (2014) The prevention of titanium-particle-induced osteolysis by OA-14 through the suppression of the p38 signaling pathway and inhibition of osteoclastogenesis. Biomaterials. https://doi.org/10.1016/j.biomaterials.2014.06.055
Tang Y, Wu X, Lei W et al (2009) TGF-Β1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. https://doi.org/10.1038/nm.1979
Nolan T, Hands RE, Bustin SA (2006) Quantification of mRNA using real-time RT-PCR. Nat Protoc. https://doi.org/10.1038/nprot.2006.236
Wang N, Feng Y, Cheung F et al (2015) A chinese medicine formula Gegen Qinlian decoction suppresses expansion of human renal carcinoma with inhibition of matrix metalloproteinase-2. Integr Cancer Ther. https://doi.org/10.1177/1534735414550036
Yu Y, Pei M, Li L (2015) Baicalin induces apoptosis in hepatic cancer cells in vitro and suppresses tumor growth in vivo. Int J Clin Exp Med 8:8958
Neve RM, Chin K, Fridlyand J et al (2006) A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. https://doi.org/10.1016/j.ccr.2006.10.008
Müller A, Homey B, Soto H et al (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature. https://doi.org/10.1038/35065016
Body JJ (2006) Individualization of bisphosphonate therapy. Breast cancer and molecular medicine. Springer, Berlin
Grav LM, Lee JS, Gerling S et al (2015) One-step generation of triple knockout CHO cell lines using CRISPR/Cas9 and fluorescent enrichment. Biotechnol J. https://doi.org/10.1002/biot.201500027
Ray A, Nkhata KJ, Cleary MP (2007) Effects of leptin on human breast cancer cell lines in relationship to estrogen receptor and HER2 status. Int J Oncol. https://doi.org/10.3892/ijo.30.6.1499
Davies MA, Kopetz S (2013) Overcoming resistance to MAPK pathway inhibitors. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djs507
Ferlay J, Soerjomataram I, Ervik M et al (2013) Fact sheets by cancer. GLOBOCAN 2012 v1.0, Cancer Incid. Mortal. Worldw. IARC CancerBase No. 11. International Agency for Research on Cancer, Lyon
Lumachi F (2014) Malignancy-related hypercalcemia: pathophysiology and diagnosis. Anticancer res 33:6036
Zajączkowska R, Kocot-Kępska M, Leppert W, Wordliczek J (2019) Bone pain in cancer patients: mechanisms and current treatment. Int J Mol Sci 20(23):6047
Calabrese V, Cornelius C, Cuzzocrea S et al (2011) Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity. Mol Aspects Med. https://doi.org/10.1016/j.mam.2011.10.007
Bottini M, Magrini A, Fadeel B, Rosato N (2016) Tackling chondrocyte hypertrophy with multifunctional nanoparticles. Gene Ther. https://doi.org/10.1038/gt.2016.33
Tait SWG, Green DR (2012) Mitochondria and cell signalling. J Cell Sci. https://doi.org/10.1242/jcs.099234
Orrenius S, Gogvadze V, Zhivotovsky B (2015) Calcium and mitochondria in the regulation of cell death. Biochem Biophys Res Commun. https://doi.org/10.1016/j.bbrc.2015.01.137
Chandra D, Liu JW, Tang DG (2002) Early mitochondrial activation and cytochrome c up-regulation during apoptosis. J Biol Chem. https://doi.org/10.1074/jbc.M207622200
Qiu JH, Asai A, Chi S et al (2000) Proteasome inhibitors induce cytochrome c-caspase-3-like protease-mediated apoptosis in cultured cortical neurons. J Neurosci. https://doi.org/10.1523/JNEUROSCI.20-01-00259.2000
Elmore SW, Oost TK, Park CM (2005) Inhibitors of anti-apoptotic proteins for cancer therapy. Annu Rep Med Chem 40:245–262
Mohan S, Abdul AB, Abdelwahab SI et al (2010) Typhonium flagelliforme induces apoptosis in CEMss cells via activation of caspase-9, PARP cleavage and cytochrome c release: Its activation coupled with G0/G1 phase cell cycle arrest. J Ethnopharmacol. https://doi.org/10.1016/j.jep.2010.07.043
García ML, Fernández A, Solas MT (2013) Mitochondria, motor neurons and aging. J Neurol Sci. https://doi.org/10.1016/j.jns.2013.03.019
Li CY, Lee JS, Ko YG et al (2000) Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J Biol Chem. https://doi.org/10.1074/jbc.M906383199
Kroemer G (2001) B709 mitochondrial control of cell death. Sci World J 1:48
Berndt S, Gurevich VV, Gurevich EV (2017) Arrestins in cell death. The structural basis of arrestin functions. Springer, Cham
Chau BN, Chen TT, Wan YY et al (2004) Tumor necrosis factor alpha-induced apoptosis requires p73 and c-ABL activation downstream of RB degradation. Mol Cell Biol. https://doi.org/10.1128/MCB.24.10.4438-4447.2004
Kamp DW, Panduri V, Weitzman SA, Chandel N (2002) Asbestos-induced alveolar epithelial cell apoptosis: role of mitochondrial dysfunction caused by iron-derived free radicals. Mol Cell Biochem. https://doi.org/10.1023/A:1015949118495
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, B., Huang, T., Fang, Q. et al. Bone-protective and anti-tumor effect of baicalin in osteotropic breast cancer via induction of apoptosis. Breast Cancer Res Treat 184, 711–721 (2020). https://doi.org/10.1007/s10549-020-05904-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05904-y