Abstract
Purpose
The aim of the current study was to evaluate the effect of N-acetylcysteine (NAC) on the incidence and severity of paclitaxel-induced peripheral neuropathy (PIPN) in breast cancer patients.
Method
A prospective randomized controlled open label study was conducted on 75 breast cancer patients receiving adjuvant paclitaxel 80 mg/m2 weekly for 12 weeks. Eligible patients were randomized to either the low dose group; 1200 mg daily NAC, the high dose group; 1200 mg NAC twice daily or the control group; received paclitaxel only. The primary endpoint was the incidence of different grades of PIPN using National Cancer Institute’s common toxicity criteria for adverse event (NCI-CTCAE) while secondary endpoints were the severity of PIPN using modified total neuropathy score (mTNS), quality of life (QOL) using Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (FACT-GOG-NTX) subscale, serum nerve growth factor (NGF), and serum malondialdehyde (MDA).
Results
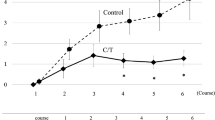
At the end of the 12-week period, the incidence of grade (2, 3) peripheral neuropathy was significantly lower in the high dose group (28.6%) compared to the low dose group (61.9%) and the control group (100%), p value < 0.001. A significant improvement in the mTNS and QOL scores was observed after 6 and 12 weeks in the high dose group and the low dose group compared to the control, p value < 0.001. Significantly higher levels of serum NGF in the high dose group and lower level of serum MDA in the high dose and the low dose group were observed.
Conclusion
Oral NAC (1200 mg once and twice daily) might reduce the incidence and severity of PIPN and improve the patients’ QOL.
Trial registry
Clinical Trial.gov registration number: NCT03492047.


Similar content being viewed by others
References
Barnard ME, Boeke CE, Tamimi RM (2015) Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim Biophys Acta 1856(1):73–85. https://doi.org/10.1016/j.bbcan.2015.06.002
Ibrahim AS, Khaled HM, Mikhail NN, Baraka H, Kamel H (2014) Cancer incidence in Egypt: results of the national population-based cancer registry program. J Cancer Epidemiol 2014:437971. https://doi.org/10.1155/2014/437971
Chew HK (2001) Adjuvant therapy for breast cancer: who should get what? West J Med 174(4):284–287. https://doi.org/10.1136/ewjm.174.4.284
Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, Wolff AC, Sledge GW Jr, Wood WC, Davidson NE (2008) Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358(16):1663–1671. https://doi.org/10.1056/NEJMoa0707056
Brami C, Bao T, Deng G (2016) Natural products and complementary therapies for chemotherapy-induced peripheral neuropathy: a systematic review. Crit Rev Oncol Hematol 98:325–334. https://doi.org/10.1016/j.critrevonc.2015.11.014
Bao T, Basal C, Seluzicki C, Li SQ, Seidman AD, Mao JJ (2016) Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res Treat 159(2):327–333. https://doi.org/10.1007/s10549-016-3939-0
Ewertz M, Qvortrup C, Eckhoff L (2015) Chemotherapy-induced peripheral neuropathy in patients treated with taxanes and platinum derivatives. Acta Oncol 54(5):587–591. https://doi.org/10.3109/0284186x.2014.995775
Bakogeorgos M, Georgoulias V (2017) Risk-reduction and treatment of chemotherapy-induced peripheral neuropathy. Expert Rev Anticancer Ther 17(11):1045–1060. https://doi.org/10.1080/14737140.2017.1374856
Grisold W, Grisold A (2017) Chemotherapy-induced peripheral neuropathy: limitations in current prophylactic/therapeutic strategies and directions for future research. Curr Med Res Opin 33(7):1291–1292. https://doi.org/10.1080/03007995.2017.1314263
Carvalho LF, Silva AMF, Carvalho AA (2017) The use of antioxidant agents for chemotherapy-induced peripheral neuropathy treatment in animal models. Clin Exp Pharmacol Physiol 44(10):971–979. https://doi.org/10.1111/1440-1681.12803
Areti A, Yerra VG, Naidu V, Kumar A (2014) Oxidative stress and nerve damage: role in chemotherapy induced peripheral neuropathy. Redox Biol 2:289–295. https://doi.org/10.1016/j.redox.2014.01.006
Ghoreishi Z, Esfahani A, Djazayeri A, Djalali M, Golestan B, Ayromlou H, Hashemzade S, Asghari Jafarabadi M, Montazeri V, Keshavarz SA, Darabi M (2012) Omega-3 fatty acids are protective against paclitaxel-induced peripheral neuropathy: a randomized double-blind placebo controlled trial. BMC Cancer 12:355. https://doi.org/10.1186/1471-2407-12-355
Argyriou AA, Chroni E, Koutras A, Ellul J, Papapetropoulos S, Katsoulas G, Iconomou G, Kalofonos HP (2005) Vitamin E for prophylaxis against chemotherapy-induced neuropathy: a randomized controlled trial. Neurology 64(1):26–31. https://doi.org/10.1212/01.wnl.0000148609.35718.7d
Gao W, Zan Y, Wang ZJ, Hu XY, Huang F (2016) Quercetin ameliorates paclitaxel-induced neuropathic pain by stabilizing mast cells, and subsequently blocking PKCepsilon-dependent activation of TRPV1. Acta Pharmacol Sin 37(9):1166–1177. https://doi.org/10.1038/aps.2016.58
Griffiths LA, Flatters SJ (2015) Pharmacological modulation of the mitochondrial electron transport chain in paclitaxel-induced painful peripheral neuropathy. J Pain 16(10):981–994. https://doi.org/10.1016/j.jpain.2015.06.008
Hershman DL, Lacchetti C, Dworkin RH, Smith EML, Bleeker J, Cavaletti G, Chauhan C, Gavin P, Lavino A, Lustberg MB, Paice J, Schneider B, Smith ML, Smith T, Terstriep S, Wagner-Johnston N, Bak K, Loprinzi CL (2014) Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 32(18):1941–1967. https://doi.org/10.1200/jco.2013.54.0914
Li J, Xu L, Deng X, Jiang C, Pan C, Chen L, Han Y, Dai W, Hu L, Zhang G, Cheng Z, Liu W (2016) N-Acetyl-cysteine attenuates neuropathic pain by suppressing matrix metalloproteinases. Pain 157(8):1711–1723. https://doi.org/10.1097/j.pain.0000000000000575
Park SA, Choi KS, Bang JH, Huh K, Kim SU (2000) Cisplatin-induced apoptotic cell death in mouse hybrid neurons is blocked by antioxidants through suppression of cisplatin-mediated accumulation of p53 but not of Fas/Fas ligand. J Neurochem 75(3):946–953. https://doi.org/10.1046/j.1471-4159.2000.0750946.x
Lin PC, Lee MY, Wang WS, Yen CC, Chao TC, Hsiao LT, Yang MH, Chen PM, Lin KP, Chiou TJ (2006) N-Acetylcysteine has neuroprotective effects against oxaliplatin-based adjuvant chemotherapy in colon cancer patients: preliminary data. Support Care Cancer 14(5):484–487. https://doi.org/10.1007/s00520-006-0018-9
Coles LD, Tuite PJ, Öz G, Mishra UR, Kartha RV, Sullivan KM, Cloyd JC, Terpstra M (2018) Repeated-dose oral N-acetylcysteine in Parkinson's disease: pharmacokinetics and effect on brain glutathione and oxidative stress. J Clin Pharmacol 58(2):158–167
Zhang Y, Gong S, He L, Zhou M, Guo J, Hoke A, Zhu C (2017) Nerve growth factor for neuropathic pain. Cochrane Database Syst Rev 2017(11):CD012800
Chang DS, Hsu E, Hottinger DG, Cohen SP (2016) Anti-nerve growth factor in pain management: current evidence. J Pain Res 9:373
De Santis S, Pace A, Bove L, Cognetti F, Properzi F, Fiore M, Triaca V, Savarese A, Simone MD, Jandolo BJ (2000) Patients treated with antitumor drugs displaying neurological deficits are characterized by a low circulating level of nerve growth factor. Clin Cancer Res 6(1):90–95
Smith EM, Beck SL, Cohen J (2008) The total neuropathy score: a tool for measuring chemotherapy-induced peripheral neuropathy. Oncol Nurs Forum 35(1):96–102. https://doi.org/10.1188/08.onf.96-102
Wampler MA, Miaskowski C, Hamel K, Byl N, Rugo H, Topp KS (2006) The modified total neuropathy score: a clinically feasible and valid measure of taxane-induced peripheral neuropathy in women with breast cancer. J Support Oncol 4(8):W9–W16
Huang HQ, Brady MF, Cella D, Fleming G (2007) Validation and reduction of FACT/GOG-Ntx subscale for platinum/paclitaxel-induced neurologic symptoms: a gynecologic oncology group study. Int J Gynecol Cancer 17(2):387–393. https://doi.org/10.1111/j.1525-1438.2007.00794.x
Calhoun EA, Welshman E, Chang C-H, Lurain J, Fishman D, Hunt T, Cella DJ (2003) Psychometric evaluation of the functional assessment of cancer therapy/gynecologic oncology group—neurotoxicity (Fact/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer 13(6):741–748
Tsikas D, Rothmann S, Schneider JY, Suchy M-T, Trettin A, Modun D, Stuke N, Maassen N, Frölich JC (2016) Development, validation and biomedical applications of stable-isotope dilution GC–MS and GC–MS/MS techniques for circulating malondialdehyde (MDA) after pentafluorobenzyl bromide derivatization: MDA as a biomarker of oxidative stress and its relation to 15 (S)-8-iso-prostaglandin F2α and nitric oxide (NO). J Chromatogr 1019:95–111
Singh Z, Karthigesu IP, Singh P, Rupinder KJ (2014) Use of malondialdehyde as a biomarker for assessing oxidative stress in different disease pathologies: a review. Iran J Public Health 43(3):7–16
Carr AJ, Higginson IJ (2001) Are quality of life measures patient centred? BMJ 322(7298):1357–1360. https://doi.org/10.1136/bmj.322.7298.1357
Tofthagen C, Visovsky C, Dominic S, McMillan S (2019) Neuropathic symptoms, physical and emotional well-being, and quality of life at the end of life. Support Care Cancer 27(9):3357–3364. https://doi.org/10.1007/s00520-018-4627-x
Cella D, Peterman A, Hudgens S, Webster K, Socinski MA (2003) Measuring the side effects of taxane therapy in oncology: the functional assesment of cancer therapy-taxane (FACT-taxane). Cancer 98(4):822–831. https://doi.org/10.1002/cncr.11578
Crevenna R, Ashbury FD (2018) Physical interventions for patients suffering from chemotherapy-induced polyneuropathy. Support Care Cancer 26(4):1017–1018. https://doi.org/10.1007/s00520-018-4071-y
Youk J, Kim YS, Lim JA, Shin DY, Koh Y, Lee ST, Kim I (2017) Depletion of nerve growth factor in chemotherapy-induced peripheral neuropathy associated with hematologic malignancies. PLoS ONE 12(8):e0183491. https://doi.org/10.1371/journal.pone.0183491
Decroli E, Manaf A, Syahbuddin S, Syafrita Y, Dillasamola D (2019) The correlation between malondialdehyde and nerve growth factor serum level with diabetic peripheral neuropathy score. Open Access Maced J Med Sci 7(1):103
Cavaletti G, Bogliun G, Marzorati L, Zincone A, Piatti M, Colombo N, Franchi D, La Presa MT, Lissoni A, Buda A, Fei F, Cundari S, Zanna C (2004) Early predictors of peripheral neurotoxicity in cisplatin and paclitaxel combination chemotherapy. Ann Oncol 15(9):1439–1442. https://doi.org/10.1093/annonc/mdh348
Binder CJ (2017) Lipid modification and lipid peroxidation products in innate immunity and inflammation. Biochim Biophys Acta Mol Cell Biol Lipids 1862(4):369–370. https://doi.org/10.1016/j.bbalip.2017.01.006
Kim ST, Chung YH, Lee HS, Chung SJ, Lee JH, Sohn UD, Shin YK, Park ES, Kim HC, Bang JS, Jeong JH (2015) Protective effects of phosphatidylcholine on oxaliplatin-induced neuropathy in rats. Life Sci 130:81–87. https://doi.org/10.1016/j.lfs.2015.03.013
Acknowledgements
This paper isn’t funded by any funding bodies or any pharmaceutical companies. Authors would like to acknowledge patient and their families for participation in this study
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
The study was done according to the ethical standards of Helsinki declaration and its later amendments.
Informed consent
Written informed consents were obtained from all participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khalefa, H.G., Shawki, M.A., Aboelhassan, R. et al. Evaluation of the effect of N-acetylcysteine on the prevention and amelioration of paclitaxel-induced peripheral neuropathy in breast cancer patients: a randomized controlled study. Breast Cancer Res Treat 183, 117–125 (2020). https://doi.org/10.1007/s10549-020-05762-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05762-8