Abstract
Purpose
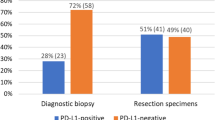
PD-L1 expression is a predictive biomarker for anti-PD-L1 immunotherapy in triple negative breast cancer (TNBC). In the neoadjuvant setting, immunohistochemical (IHC) evaluation of PD-L1 expression can only be performed on small tissue biopsies. In our study we investigated heterogeneity of PD-L1 expression in TNBC, and how reliably PD-L1 expression in small tissue samples reflects PD-L1 expression in larger tumor sections in TNBC.
Methods
Tissue microarrays (TMAs) were constructed from surgical specimens of 110 patients with TNBC. TMAs contained 4 cores (1 mm in diameter) per patient. To evaluate PD-L1 expression, TMAs were stained with PD-L1 IHC 22C3 PharmDx. Single-core PD-L1 expression was compared to overall PD-L1 expression of each patient’s tumor, to ascertain how often small samples of tumor tissue show the same PD-L1 expression as larger tumor samples.
Results
Our study found substantial heterogeneity of PD-L1 expression between different TMA cores from the same patient. Heterogeneity was greater in immune cells (ICs) than in tumor cells, in large part due to the uneven distribution of ICs in the tumor. For IC PD-L1 expression, we found that sensitivity can be as low as 0.81 for detecting PD-L1 expression at the 1% threshold most commonly used in breast cancer. Negative predictive value for ICs was 0.7.
Conclusions
There is substantial heterogeneity of PD-L1 expression between small tissue samples from the same TNBC tumor, especially for IC expression. This poses challenges for evaluation of PD-L1 expression in the neoadjuvant setting. Negative biopsies should prompt further investigation, and multiple biopsies might be necessary.



Similar content being viewed by others
References
Reis-Filho JS, Tutt ANJ (2007) Triple negative tumours: a critical review. Histopathology 52:108–118. https://doi.org/10.1200/JCO.2007.13.1748
Bianchini G, Balko JM, Mayer IA et al (2016) Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 13:674–690. https://doi.org/10.1038/nrclinonc.2016.66
Schmid P, Adams S, Rugo HS et al (2018) Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379:2108–2121. https://doi.org/10.1056/NEJMoa1809615
Dill EA, Gru AA, Atkins KA et al (2017) PD-L1 expression and intratumoral heterogeneity across breast cancer subtypes and stages: an assessment of 245 primary and 40 metastatic tumors. Am J Surg Pathol 41:334–342. https://doi.org/10.1097/PAS.0000000000000780
Yuan C, Liu Z, Yu Q et al (2019) Expression of PD-1/PD-L1 in primary breast tumours and metastatic axillary lymph nodes and its correlation with clinicopathological parameters. Sci Rep 9(1):14356. https://doi.org/10.1038/s41598-019-50898-3
Manson QF, Schrijver WAME, ter Hoeve ND et al (2019) Frequent discordance in PD-1 and PD-L1 expression between primary breast tumors and their matched distant metastases. Clin Exp Metastasis 36:29–37. https://doi.org/10.1007/s10585-018-9950-6
Ogiya R, Niikura N, Kumaki N et al (2016) Comparison of tumor-infiltrating lymphocytes between primary and metastatic tumors in breast cancer patients. Cancer Sci 107:1730–1735. https://doi.org/10.1111/cas.13101
Cimino-Mathews A, Thompson E, Taube JM et al (2016) PD-L1 (B7–H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol 47:52–63. https://doi.org/10.1016/j.humpath.2015.09.003
Munari E, Zamboni G, Lunardi G et al (2018) PD-L1 expression heterogeneity in non-small cell lung cancer: defining criteria for harmonization between biopsy specimens and whole sections. J Thorac Oncol 13:1113–1120. https://doi.org/10.1016/j.jtho.2018.04.017
Camp RL, Charette LA, Rimm DL (2000) Validation of tissue microarray technology in breast carcinoma. Lab Investig 80:1943–1949. https://doi.org/10.1038/labinvest.3780204
Narayan P, Wahby S, Gao JJ et al (2020) FDA approval summary: atezolizumab plus paclitaxel protein-bound for the treatment of patients with advanced or metastatic TNBC whose tumors express PD-L1. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-19-3545
Abstract (2019) Abstracts: 31st European Congress of Pathology (2019). Virchows Arch 475:1–436. https://doi.org/10.1007/s00428-019-02631-8
Chen DS, Mellman I (2017) Elements of cancer immunity and the cancer-immune set point. Nature 541(7637):321–330. https://doi.org/10.1038/nature21349
Rimm DL, Han G, Taube JM et al (2017) A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non–small cell lung cancer. JAMA Oncol 3:1051–1058. https://doi.org/10.1001/jamaoncol.2017.0013
Gniadek TJ, Li QK, Tully E et al (2017) Heterogeneous expression of PD-L1 in pulmonary squamous cell carcinoma and adenocarcinoma: implications for assessment by small biopsy. Mod Pathol 30:530–538. https://doi.org/10.1038/modpathol.2016.213
Gagné A, Enlow W, Pigeon M-A et al (2018) Comprehensive assessment of PD-L1 staining heterogeneity in pulmonary adenocarcinomas using tissue microarrays: impact of the architecture pattern and the number of cores. Am J Surg Pathol 42:687–694. https://doi.org/10.1097/PAS.0000000000001013
Kitazono S, Fujiwara Y, Tsuta K et al (2015) Reliability of small biopsy samples compared with resected specimens for the determination of programmed death-ligand 1 expression in non-small-cell lung cancer. Clin Lung Cancer 16:385–390. https://doi.org/10.1016/j.cllc.2015.03.008
Haragan A, Field JK, Davies MPA et al (2019) Heterogeneity of PD-L1 expression in non-small cell lung cancer: implications for specimen sampling in predicting treatment response. Lung Cancer 134:79–84. https://doi.org/10.1016/j.lungcan.2019.06.005
Gradecki SE, Grange JS, Stelow EB (2018) Concordance of PD-L1 expression between core biopsy and resection specimens of non-small cell lung cancer. Am J Surg Pathol 42:1090–1094. https://doi.org/10.1097/PAS.0000000000001085
Munari E, Zamboni G, Marconi M et al (2017) PD-L1 expression heterogeneity in non-small cell lung cancer: evaluation of small biopsies reliability. Oncotarget 8:90123–90131
Li C, Huang C, Mok TS et al (2017) Comparison of 22C3 PD-L1 expression between surgically resected specimens and paired tissue microarrays in non-small cell lung cancer. J Thorac Oncol 12:1536–1543. https://doi.org/10.1016/j.jtho.2017.07.015
Acknowledgements
The authors wish to thank laboratory technicians Christina Grønhøj and Dorte Skriver-Jensen, and the laboratory of the Pathology Department, Herlev and Gentofte hospital for their work with TMA production and immunohistochemical staining.
Funding
The study was funded by the Pathology Department, Herlev and Gentofte Hospital, the Research Foundation of Herlev and Gentofte Hospital, Bent Bøgh and Inge Bøgh Foundation, Axel Muusfeldt Foundation, Mogens Ambt Balslev Memorial Foundation, Andersen Isted Foundation and HC Bechgaard and Mary Bechgaard Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Elisabeth Specht Stovgaard. The first draft of the manuscript was written by Elisabeth Specht Stovgaard and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This research study was conducted retrospectively using material obtained for clinical purposes. The study was approved by the Danish Ethics Committee (Project Number H-15015306).
Informed consent
The material used in this study had previously been obtained for clinical purposes, and patients had been informed that the material could be used for research purposes unless they actively registered in The Danish Registry for Use of Tissue. The registry was consulted before use of material, and no patients in the study were registered.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stovgaard, E.S., Bokharaey, M., List-Jensen, K. et al. PD-L1 diagnostics in the neoadjuvant setting: implications of intratumoral heterogeneity of PD-L1 expression in triple negative breast cancer for assessment in small biopsies. Breast Cancer Res Treat 181, 553–560 (2020). https://doi.org/10.1007/s10549-020-05655-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05655-w