Abstract
Purpose
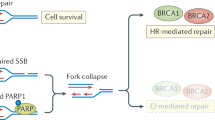
PARP-3 is member of the PARP family of poly (ADP-ribose) polymerases involved in ADPribosylation. PARPs are involved in the basic mechanisms of DNA repair. PARP3, a critical player for efficient mitotic progression, is required for the stabilization of the mitotic spindle by regulation of the mitotic components, NuMA and Tankyrase 1.
Methods
The sensitization effect of vinorelbine on PARP3 inhibition-induced cytotoxicity was assessed by the SRB assay. The contribution of programed cell death and cell cycle arrest to the sensitization effect were determined by assessing changes in Annexin V, a marker of apoptosis. Alterations in cell cycle progression were assessed by cell cycle analysis. We used immunofluorescence to assess the effect of vinorelbine and/or PARP3 inhibitors on tubulin and microtubule depolarization. The PARP3 chemiluminescent assay kit was used for PARP3 activity.
Results
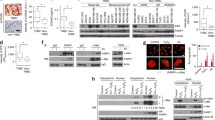
PARP3 inhibitors sensitize breast cancer cells to vinorelbine, a vinca alkaloid used in the treatment of metastatic breast cancer. Olaparib which was originally described as a PARP1 and 2 inhibitor has recently been shown to be a potent PARP3 inhibitor while ME0328 is a more selective PARP3 inhibitor. The combination of vinorelbine with nontoxic concentrations of ME0328 or olaparib reduces vinorelbine resistance by 10 and 17 fold, respectively, potentiating vinorelbine-induced arrest at the G2/M boundary. In addition, PARP3 inhibition potentiates vinorelbine interaction with tubulin. Furthermore, olaparib or ME0328 potentiates vinorelbine-induced PARP3 inhibition, mitotic arrest, and apoptosis.
Conclusion
Our results indicated this approach with PARP3 inhibitors and vinorelbine is unique and promising for breast cancer patients with metastases. This combination could significantly increase the survival of breast cancer patients with metastases.






Similar content being viewed by others
References
Langelier MF, Riccio AA, Pascal JM (2014) PARP-2 and PARP-3 are selectively activated by 5 phosphorylated DNA breaks through an allosteric regulatory mechanism shared with PARP-1. Nucleic Acids Res 42(12):7762–7775
Krishnakumar R, Kraus WL (2010) The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets. Mol Cell 39(1):8–24
Boehler C, Dantzer F (2011) PARP-3, a DNA-dependent PARP with emerging roles in double-strand break repair and mitotic progression. Cell Cycle 10(7):1023–1024
De Vos M, Schreiber V, Dantzer F (2012) The diverse roles and clinical relevance of PARPs in DNA damage repair: current state of the art. Biochem Pharmacol 84(2):137–146
Robert I, Gaudot L, Rogier M, Heyer V, Noll A, Dantzer F et al (2015) Parp3 negatively regulates immunoglobulin class switch recombination. PLoS Genet 11(5):e1005240
Beck C, Boehler C, Guirouilh Barbat J, Bonnet ME, Illuzzi G, Ronde P et al (2014) PARP3 affects the relative contribution of homologous recombination and nonhomologous end-joining pathways. Nucleic Acids Res 42(9):5616–5632
Boehler C, Gauthier LR, Mortusewicz O, Biard DS, Saliou JM, Bresson A et al (2011) Poly(ADP-ribose) polymerase 3 (PARP3), a newcomer in cellular response to DNA damage and mitotic progression. Proc Natl Acad Sci U S A 108(7):2783–2788
Rulten SL, Fisher AE, Robert I, Zuma MC, Rouleau M, Ju L et al (2011) PARP-3 and APLF function together to accelerate nonhomologous end-joining. Mol Cell 41(1):33–45
Davidson D, Amrein L, Panasci L, Aloyz R. Small, Molecules (2013) Inhibitors of DNA-PK, targeting DNA repair, and beyond. Front Pharmacol 4:5
Amrein L, Davidson D, Shawi M, Petruccelli LA, Miller WH Jr, Aloyz R et al (2011) Dual inhibition of the homologous recombinational repair and the nonhomologous end-joining repair pathways in chronic lymphocytic leukemia therapy. Leuk Res 35(8):1080–1086
Penning TD, Zhu GD, Gandhi VB, Gong J, Liu X, Shi Y et al (2009) Discovery of the Poly(ADP-ribose) polymerase (PARP) inhibitor 2-[(R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-4-carboxamide (ABT-888) for the treatment of cancer. J Med Chem 52(2):514–523
Augustin A, Spenlehauer C, Dumond H, Menissier-De Murcia J, Piel M, Schmit AC et al (2003) PARP-3 localizes preferentially to the daughter centriole and interferes with the G1/S cell cycle progression. J Cell Sci 116(Pt 8):1551–1562
Fernandez-Marcelo T, Frias C, Pascua I, de Juan C, Head J, Gomez A et al (2014) Poly (ADP-ribose) polymerase 3 (PARP3), a potential repressor of telomerase activity. J Exp Clin Cancer Res 33:19
Sonnenblick A, de Azambuja E, Azim HA Jr, Piccart M (2015) An update on PARP inhibitors–moving to the adjuvant setting. Nat Rev Clin Oncol 12:27–41
Bundred N, Gardovskis J, Jaskiewicz J, Eglitis J, Paramonov V, McCormack P et al (2013) Evaluation of the pharmacodynamics and pharmacokinetics of the PARP inhibitor olaparib: a phase I multicentre trial in patients scheduled for elective breast cancer surgery. Invest New Drugs 31(4):949–958
Nijman SM (2011) Synthetic lethality: general principles, utility and detection using genetic screens in human cells. FEBS Lett 585(1):1–6
Hernandez-Blanquisett A, Touya D, Strasser-Weippl K, Ruiz R, St Louis J, Goss P (2016) Current and emerging therapies of HER2-positive metastatic breast cancer. Breast 29:170–177
Ferrario C, Strepponi I, Esfahani K, Charamis H, Langleben A et al. (2016) Phase I/II trial of sorafenib in combination with vinorelbine as first-line chemotherapy for metastatic breast cancer. PLoS ONE 11(12):e0167906
Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN et al (2016) Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology guideline. J Clin Oncol 34(25):3069–3103
Faller BA, Pandit TN (2011) Safety and efficacy of vinorelbine in the treatment of non-small cell lung cancer. Clin Med Insights Oncol 5:131–144
Coderch C, Morreale A, Gago F (2012) Tubulin-based structure-affinity relationships for antimitotic Vinca alkaloids. Anticancer Agents Med Chem 12(3):219–225
Klotz DM, Nelson SA, Kroboth K, Newton IP, Radulescu S, Ridgway RA et al (2012) The microtubule poison vinorelbine kills cells independently of mitotic arrest and targets cells lacking the APC tumour suppressor more effectively. J Cell Sci 125(Pt 4):887–895
Wendell KL, Wilson L, Jordan MA (1993) Mitotic block in HeLa cells by vinblastine: ultrastructural changes in kinetochore-microtubule attachment and in centrosomes. J Cell Sci 104(Pt 2):261–274
Xu YC, Wang HX, Tang L, Ma Y, Zhang FC (2013) A systematic review of vinorelbine for the treatment of breast cancer. Breast J 19(2):180–188
Lindgren AE, Karlberg T, Thorsell AG, Hesse M, Spjut S, Ekblad T et al (2013) PARP inhibitor with selectivity toward ADP-ribosyltransferase ARTD3/PARP3. ACS Chem Biol 8(8):1698–1703
Robert M, Frenel JS, Gourmelon C, Patsouris A, Augereau P, Campone M (2017) Olaparib for the treatment of breast cancer. Expert Opin Investig Drugs 26(6):751–759
Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN et al (2010) Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 376(9737):235–244
Chan SL, Mok T (2010) PARP inhibition in BRCA-mutated breast and ovarian cancers. Lancet 376(9737):211–213
Arun B, Akar U, Gutierrez-Barrera AM, Hortobagyi GN, Ozpolat B (2015) The PARP inhibitor AZD2281 (Olaparib) induces autophagy/mitophagy in BRCA1 and BRCA2 mutant breast cancer cells. Int J Oncol 47(1):262–268
Rizvi W, Truong P, Truong Q (2017) Metastatic breast cancer with BRCA mutation discovered by next-generation sequencing responding to olaparib. Cureus 9(6):e1337
Senra JM, Telfer BA, Cherry KE, McCrudden CM, Hirst DG, O’Connor MJ et al (2011) Inhibition of PARP-1 by olaparib (AZD2281) increases the radiosensitivity of a lung tumor xenograft. Mol Cancer Ther 10(10):1949–1958
Chen A (2011) PARP inhibitors: its role in treatment of cancer. Chin J Cancer 30(7):463–471
Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N et al (2017) Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 377(6):523–533
Davidson D, Wang Y, Aloyz R, Panasci L (2013) The PARP inhibitor ABT-888 synergizes irinotecan treatment of colon cancer cell lines. Invest New Drugs 31(2):461–468
Vichai V, Kirtikara K (2006) Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc 1(3):1112–1116
Davidson D, Coulombe Y, Martinez-Marignac VL, Amrein L, Grenier J, Hodkinson K et al (2012) Irinotecan and DNA-PKcs inhibitors synergize in killing of colon cancer cells. Invest New Drugs 30(3):1248–1256
Davidson D, Grenier J, Martinez-Marignac V, Amrein L, Shawi M, Tokars M et al (2012) Effects of the novel DNA dependent protein kinase inhibitor, IC486241, on the DNA damage response to doxorubicin and cisplatin in breast cancer cells. Invest New Drugs 30(4):1736–1742
Xu ZY, Loignon M, Han FY, Panasci L, Aloyz R (2005) Xrcc3 induces cisplatin resistance by stimulation of Rad51-related recombinational repair, S-phase checkpoint activation, and reduced apoptosis. J Pharmacol Exp Ther 314(2):495–505
Wang YR, Xu Y, Jiang ZZ, Guerram M, Wang B et al. (2015) Deoxypodophyllotoxin induces G2/M cell cycle arrest and apoptosis in SGC-7901 cells and inhibits tumor growth in vivo. Molecules 20(1):1661–1675
Chiu WH, Luo SJ, Chen CL, Cheng JH, Hsieh CY, Wang CY et al (2012) Vinca alkaloids cause aberrant ROS-mediated JNK activation, Mcl-1 downregulation, DNA damage, mitochondrial dysfunction, and apoptosis in lung adenocarcinoma cells. Biochem Pharmacol 83(9):1159–1171
Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nat Rev Cancer 4(4):253–265
Okouneva T, Hill BT, Wilson L, Jordan MA (2003) The effects of vinflunine, vinorelbine, and vinblastine on centromere dynamics. Mol Cancer Ther 2(5):427–436
Fujita H, Yoshino Y, Chiba N (2016) Regulation of the centrosome cycle. Mol Cell Oncol 3(2):e1075643
Oplustil O’Connor, Rulten L, Cranston SL, Odedra AN, Brown R (2016) H, et al. The PARP inhibitor ZD2461 provides insights into the role of PARP3 inhibition for both synthetic lethality and tolerability with chemotherapy in preclinical models. Cancer Res 76(20):6084–6094
Funding
The Cancer Research Society supported this investigation. Grant Number: 01713.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The experiments comply with the current laws of Canada in which they were performed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharif-Askari, B., Amrein, L., Aloyz, R. et al. PARP3 inhibitors ME0328 and olaparib potentiate vinorelbine sensitization in breast cancer cell lines. Breast Cancer Res Treat 172, 23–32 (2018). https://doi.org/10.1007/s10549-018-4888-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4888-6