Abstract
Purpose
The new eighth edition TNM classification by the AJCC for breast cancer (BC) incorporates biologic factors and gene expression prognostic panels, in addition to traditional anatomic factors. In this study, we evaluated the prognostic value of this new staging system compared to the previous AJCC 7th edition staging system.
Methods
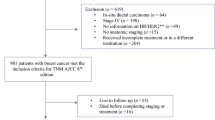
We conducted a retrospective analysis of women with stage I, II, or III BC who underwent curative surgery with/without adjuvant systemic therapy at Samsung Medical Center between July 2004 and December 2008.
Results
Of 3,208 BCs, this study was analyzed using the information of 2,790 BC patients. Hormone receptor-positive (HR+) and human epidermal growth factor 2 (HER2)− BCs were observed in 62.9% of BCs, HR+/ HER2+ in 9.3%, HR−/HER2− in 17.0%, and HR−/HER2+ in 10.8%. In survival analysis, we observed 245 distant recurrences and 198 deaths caused by BC progression. The median follow-up duration was 116.2 months. 10-year disease-specific survival (DSS) rates according to the AJCC 7th edition criteria were 97.2% of stage IA, 100% of IB, 94.9% of IIA, 87.9% of IIB, 86.4% of IIIA, 95.7% of IIIB, and 65.7% of IIIC (p < 0.001). After applying 8th edition criteria, the 10-year DSS rates were 98.1% of stage IA, 97.7% of IB, 93.8% of IIA, 92.7% of IIB, 88.2% of IIIA, 80.8% of IIIB, and 70.3% of IIIC (p < 0.001).
Conclusions
The AJCC 8th edition clinical staging system provides a good prognostic value and addresses the weakness of the AJCC 7th edition, which uses only anatomical pathologic staging.




Similar content being viewed by others
References
Edge SBCC. (2009) The AJCC Cancer Staging Manual. 7th edn. Springer, New York
Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA (2010) Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol 11:174–183. https://doi.org/10.1016/S1470-2045(09)70262-1
Elston CW, Ellis IO (2002) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 41:154–161
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19:403–410
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Perez EA, Olson JA Jr, Zujewski J, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin P, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Atkins JN, Berenberg JL, Sledge GW (2015) Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 373:2005–2014. https://doi.org/10.1056/NEJMoa1510764
Cardoso F, van’t Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, Pierga JY, Brain E, Causeret S, DeLorenzi M, Glas AM, Golfinopoulos V, Goulioti T, Knox S, Matos E, Meulemans B, Neijenhuis PA, Nitz U, Passalacqua R, Ravdin P, Rubio IT, Saghatchian M, Smilde TJ, Sotiriou C, Stork L, Straehle C, Thomas G, Thompson AM, van der Hoeven JM, Vuylsteke P, Bernards R, Tryfonidis K, Rutgers E, Piccart M, Investigators M (2016) 70-Gene Signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 375:717–729. https://doi.org/10.1056/NEJMoa1602253
Dubsky P, Filipits M, Jakesz R, Rudas M, Singer CF, Greil R, Dietze O, Luisser I, Klug E, Sedivy R, Bachner M, Mayr D, Schmidt M, Gehrmann MC, Petry C, Weber KE, Kronenwett R, Brase JC, Gnant M, Austrian B, Colorectal Cancer Study G (2013) EndoPredict improves the prognostic classification derived from common clinical guidelines in ER-positive, HER2-negative early breast cancer. Ann Oncol 24:640–647. https://doi.org/10.1093/annonc/mds334
Nielsen TO, Parker JS, Leung S, Voduc D, Ebbert M, Vickery T, Davies SR, Snider J, Stijleman IJ, Reed J, Cheang MC, Mardis ER, Perou CM, Bernard PS, Ellis MJ (2010) A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res 16:5222–5232. https://doi.org/10.1158/1078-0432.CCR-10-1282
Sgroi DC, Sestak I, Cuzick J, Zhang Y, Schnabel CA, Schroeder B, Erlander MG, Dunbier A, Sidhu K, Lopez-Knowles E, Goss PE, Dowsett M (2013) Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol 14:1067–1076. https://doi.org/10.1016/S1470-2045(13)70387-5
Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, Sahmoud T, Group AT (2002) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 359:2131–2139
Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Colleoni M, Lang I, Del Mastro L, Smith I, Chirgwin J, Nogaret JM, Pienkowski T, Wardley A, Jakobsen EH, Price KN, Goldhirsch A (2007) Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol 25:486–492. https://doi.org/10.1200/JCO.2006.08.8617
Trialists’ Collaborative EBreastC, Davies G, Godwin C, Gray J, Clarke R, Cutter M, Darby D, McGale S, Pan P, Taylor HC, Wang C, Dowsett YC, Ingle M, Peto J R (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378:771–784. https://doi.org/10.1016/S0140-6736(11)60993-8
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353:1673–1684. https://doi.org/10.1056/NEJMoa052122
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC, American Society of Clinical O, College of American P (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med 134:e48-72. https://doi.org/10.1043/1543-2165-134.7.e48
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF, American Society of Clinical O, College of American P (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997–4013. https://doi.org/10.1200/JCO.2013.50.9984
Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, Weaver DL, Winchester DJ, Hortobagyi GN (2017) Breast cancer-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 67:290–303. https://doi.org/10.3322/caac.21393
Abdel-Rahman O (2017) Validation of the 8th AJCC prognostic staging system for breast cancer in a population-based setting. Breast Cancer Res Treat. https://doi.org/10.1007/s10549-017-4577-x
Weiss A, Chavez-MacGregor M, Lichtensztajn DY, Yi M, Tadros A, Hortobagyi GN, Giordano SH, Hunt KK, Mittendorf EA (2017) Validation study of the American Joint Committee on Cancer eighth edition prognostic stage compared With the anatomic stage in breast cancer. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2017.4298
Hu H, Wei W, Yi X, Xin L, Liu Y (2017) A Retrospective analysis of clinical utility of AJCC 8th edition cancer staging system for breast cancer. World J Oncol 8:71–75. https://doi.org/10.14740/wjon1039e
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflicts of interest.
Ethical approval
The experiments comply with the current laws of the country in which they were performed.
Rights and permissions
About this article
Cite this article
Kim, JY., Lim, J.E., Jung, H.H. et al. Validation of the new AJCC eighth edition of the TNM classification for breast cancer with a single-center breast cancer cohort. Breast Cancer Res Treat 171, 737–745 (2018). https://doi.org/10.1007/s10549-018-4858-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4858-z