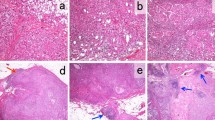
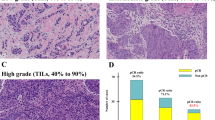
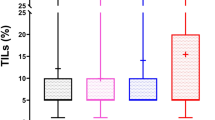
Abstract
Purpose
The therapeutic effect of systemic treatment for breast cancer (BC) generally depends on its intrinsic subtypes. In addition, tumor infiltrating lymphocytes (TILs) are considered to be an independent factor for tumor shrinkage and disease prognosis. High TILs at baseline or after primary systemic chemotherapy are reported to be associated with better survival in triple-negative or human epithelial growth factor receptor 2 (HER2)-positive BCs. However, the prognostic value of TILs in estrogen receptor (ER)-positive and HER2-negative (ER+/HER2−) BC is still controversial.
Methods
We assessed TIL score (low, intermediate, and high) before and after primary systemic chemotherapy in every subtype of BC, and compared the clinical outcomes. Biopsy specimens of 47 triple-negative, 58 HER2+ and 91 ER+/HER2− BCs were used to assess TILs before treatment. To assess TILs after treatment, we examined residual invasive carcinoma in surgically resected samples of 28 triple-negative, 30 HER2+ and 80 ER+/HER2− BCs.
Results
A high TIL score in triple-negative BC before treatment resulted in a significantly higher proportion of pathological complete response (pCR). In contrast, ER+/HER2− BC exhibited fewer instances of pCR than other subtypes. Although not statistically significant, ER+/HER2− cases with a high TIL score also tended to achieve pCR (p = 0.088). Moreover, we revealed that low TIL BCs after chemotherapy, but not at baseline, had significantly better relapse-free survival in ER+/HER2− BC (p = 0.034).
Conclusion
Pathological examination of TILs after treatment may be a surrogate marker for prognosis in ER+/HER2− BC.





Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65(1):5–29. https://doi.org/10.3322/caac.21254
Center for Cancer Control and Information Services, National Cancer Center, Japan. http://ganjoho.jp/en/public/statistics/short_pred.html. Accessed 18 Aug 2016
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA et al (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752. https://doi.org/10.1038/35021093
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98(19):10869–10874. https://doi.org/10.1073/pnas.191367098
Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, Thürlimann B, Senn HJ, Members P: Tailoring therapies–improving the management of early breast cancer (2015) St Gallen International Expert Consensus on the primary therapy of early breast cancer 2015. Ann Oncol 26(8):1533–1546. https://doi.org/10.1093/annonc/mdv221
Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM (2007) The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 13(8):2329–2334. https://doi.org/10.1158/1078-0432.CCR-06-1109
Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T (2013) A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol 14(12):1212–1218. https://doi.org/10.1038/ni.2762
Dunn GP, Old LJ, Schreiber RD (2004) The three Es of cancer immunoediting. Annu Rev Immunol 22:329–360. https://doi.org/10.1146/annurev.immunol.22.012703.104803
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331(6024):1565–1570. https://doi.org/10.1126/science.1203486
Qin A, Coffey DG, Warren EH, Ramnath N (2016) Mechanisms of immune evasion and current status of checkpoint inhibitors in non-small cell lung cancer. Cancer Med 5(9):2567–2578. https://doi.org/10.1002/cam4.819
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8):711–723. https://doi.org/10.1056/NEJMoa1003466
Aaltomaa S, Lipponen P, Eskelinen M, Kosma VM, Marin S, Alhava E, Syrjänen K (1992) Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer 28A(4–5):859–864. https://doi.org/10.1016/0959-8049(92)90134-N
Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ et al (2014) Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 32(27):2959–2966. https://doi.org/10.1200/JCO.2013.55.0491
Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, Kellokumpu-Lehtinen PL, Bono P, Kataja V, Desmedt C et al (2014) Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 25(8):1544–1550. https://doi.org/10.1093/annonc/mdu112
Ibrahim EM, Al-Foheidi ME, Al-Mansour MM, Kazkaz GA (2014) The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis. Breast Cancer Res Treat 148(3):467–476. https://doi.org/10.1007/s10549-014-3185-2
Kaufmann M, Hortobagyi GN, Goldhirsch A, Scholl S, Makris A, Valagussa P, Blohmer JU, Eiermann W, Jackesz R, Jonat W et al (2006) Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol 24(12):1940–1949. https://doi.org/10.1200/JCO.2005.02.6187
Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C et al (2010) Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 28(1):105–113. https://doi.org/10.1200/JCO.2009.23.7370
West NR, Milne K, Truong PT, Macpherson N, Nelson BH, Watson PH (2011) Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res 13(6):R126. https://doi.org/10.1186/bcr3072
Yamaguchi R, Tanaka M, Yano A, Tse GM, Yamaguchi M, Koura K, Kanomata N, Kawaguchi A, Akiba J, Naito Y et al (2012) Tumor-infiltrating lymphocytes are important pathologic predictors for neoadjuvant chemotherapy in patients with breast cancer. Hum Pathol 43(10):1688–1694. https://doi.org/10.1016/j.humpath.2011.12.013
Ono M, Tsuda H, Shimizu C, Yamamoto S, Shibata T, Yamamoto H, Hirata T, Yonemori K, Ando M, Tamura K et al (2012) Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat 132(3):793–805. https://doi.org/10.1007/s10549-011-1554-7
Mao Y, Qu Q, Zhang Y, Liu J, Chen X, Shen K (2014) The value of tumor infiltrating lymphocytes (TILs) for predicting response to neoadjuvant chemotherapy in breast cancer: a systematic review and meta-analysis. PLoS ONE 9(12):e115103. https://doi.org/10.1371/journal.pone.0115103
Dieci MV, Criscitiello C, Goubar A, Viale G, Conte P, Guarneri V, Ficarra G, Mathieu MC, Delaloge S, Curigliano G et al (2014) Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol 25(3):611–618. https://doi.org/10.1093/annonc/mdt556
Japanese Breast Cancer Society (2012) General rules for clinical and pathological recording of breast cancer, 17th edn. Kanehara & Co., Ltd, Tokyo
Loi S, Dushyanthen S, Beavis PA, Salgado R, Denkert C, Savas P et al (2016) RAS/MAPK activation is associated with reduced tumor-infiltrating lymphocytes in triple-negative breast cancer: therapeutic cooperation between MEK and PD-1/PD-L1 immune checkpoint inhibitors. Clin Cancer Res 22(6):1499–1509. https://doi.org/10.1158/1078-0432.CCR-15-1125
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31(31):3997–4013. https://doi.org/10.1200/JCO.2013.50.9984
Hida AI, Sagara Y, Yotsumoto D, Kanemitsu S, Kawano J, Baba S, Rai Y, Oshiro Y, Aogi K, Ohi Y (2016) Prognostic and predictive impacts of tumor-infiltrating lymphocytes differ between Triple-negative and HER2-positive breast cancers treated with standard systemic therapies. Breast Cancer Res Treat 158(1):1–9. https://doi.org/10.1007/s10549-016-3848-2
Hida AI, Ohi Y (2015) Evaluation of tumor-infiltrating lymphocytes in breast cancer; proposal of a simpler method. Ann Oncol 26(11):2351. https://doi.org/10.1093/annonc/mdv363
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F et al (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26(2):259–271. https://doi.org/10.1093/annonc/mdu450
Huszno J, Nożyńska EZ, Lange D, Kołosza Z, Nowara E (2017) The association of tumor lymphocyte infiltration with clinicopathological factors and survival in breast cancer. Pol J Pathol 68(1):26–32. https://doi.org/10.5114/pjp.2017.67612
Montagna E, Vingiani A, Maisonneuve P, Cancello G, Contaldo F, Pruneri G, Colleoni M (2017) Unfavorable prognostic role of tumor-infiltrating lymphocytes in hormone-receptor positive, HER2 negative metastatic breast cancer treated with metronomic chemotherapy. Breast 34:83–88. https://doi.org/10.1016/j.breast.2017.05.009
Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, Sattar H, Wang Y, Brown NK, Greene M et al (2010) The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell 18(2):160–170. https://doi.org/10.1016/j.ccr.2010.06.014
Bianchini G, Gianni L (2014) The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol 15(2):e58–e68. https://doi.org/10.1016/S1470-2045(13)70477-7
Hamy AS, Pierga JY, Sabaila A, Laas E, Bonsang-Kitzis H, Laurent C, Vincent-Salomon A, Cottu P, Lerebours F, Rouzier R, Lae M, Reyal F (2017) Stromal lymphocyte infiltration after neoadjuvant chemotherapy is associated with aggressive residual disease and lower disease-free survival in HER2-positive breast cancer. Ann Oncol 28(9):2233–2240. https://doi.org/10.1093/annonc/mdx309
García-Martínez E, Gil GL, Benito AC, González-Billalabeitia E, Conesa MA, García García T, García-Garre E, Vicente V, de la Peña FA (2017) Tumor-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast Cancer Res 16(6):488. https://doi.org/10.1186/s13058-014-0488-5
Haricharan S, Bainbridge MN, Scheet P, Brown PH (2014) Somatic mutation load of estrogen receptor-positive breast tumors predicts overall survival: an analysis of genome sequence data. Breast Cancer Res Treat 146(1):211–220. https://doi.org/10.1007/s10549-014-2991-x
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing. This study was supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (No. 15K10077).
Funding
We do not have a financial relationship with the organization that sponsored the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the ethics committee of the Hyogo College of Medicine (approval number #1886).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Watanabe, T., Hida, A.I., Inoue, N. et al. Abundant tumor infiltrating lymphocytes after primary systemic chemotherapy predicts poor prognosis in estrogen receptor-positive/HER2-negative breast cancers. Breast Cancer Res Treat 168, 135–145 (2018). https://doi.org/10.1007/s10549-017-4575-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4575-z