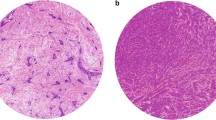
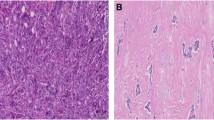
Abstract
Purpose
The primary aim of the current study is to validate the prognostic relevance of the relative amount of tumour-associated stroma, the tumour-stroma ratio, in a large cohort of primary operable breast cancer patients.
Methods
A retrospective cohort study was performed on women diagnosed and treated for primarily operable invasive breast cancer in the period from 1 January 1990 till 31 December 1999. Tumour-stroma ratio was estimated by microscopic evaluation of haematoxylin and eosin tumour slides. Two independent observers (k = 0.68) performed tumour-stroma ratio evaluation in a significant part of the cohort. The prognostic potential with respect to overall, recurrence-free and distant metastasis-free survival was evaluated.
Results
A total of n = 737 women were evaluated. Median follow-up time was 11.5 years. High stromal content was an independent prognosticator for worse overall (hazard ratio 1.56, p = 0.002, 95% confidence interval 1.18–2.05), distant metastasis-free (hazard ratio 1.52, p = 0.008, 95% confidence interval 1.12–2.06) and recurrence-free survival (hazard ratio 1.35, p = 0.046, 95% confidence interval 1.01–1.81). In subgroups of hormone receptor-positive and lymph node-negative cases, high stromal content was also an independent prognosticator for worse outcome.
Conclusion
Tumour-stroma ratio is an independent risk factor for worse overall, distant metastasis-free and recurrence-free survival in primarily operable breast cancer. However, detailed prospective studies with respect to tumour-stroma ratio are necessary to gain more insight in its prognostic potential in clinical practice.



Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Ervik M et al. (2013) GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase no. 11. Lyon, France, International Agency for Research on Cancer
Engstrom MJ, Opdahl S, Hagen AI et al (2013) Molecular subtypes, histopathological grade and survival in a historic cohort of breast cancer patients. Breast Cancer Res Treat 140(3):463–473
Soerjomataram I, Louwman MW, Ribot JG, Roukema JA, Coebergh JW (2008) An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res Treat 107(3):309–330
Pietras K, Ostman A (2010) Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res 316(8):1324–1331
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
de Kruijf EM, Sajet A, van Nes JG et al (2010) HLA-E and HLA-G expression in classical HLA class I-negative tumors is of prognostic value for clinical outcome of early breast cancer patients. J Immunol 185(12):7452–7459
Mesker WE, Liefers GJ, Junggeburt JM et al (2009) Presence of a high amount of stroma and downregulation of SMAD4 predict for worse survival for stage I-II colon cancer patients. Cell Oncol 31(3):169–178
Witkiewicz AK, Whitaker-Menezes D, Dasgupta A et al (2012) Using the “reverse Warburg effect” to identify high-risk breast cancer patients: stromal MCT4 predicts poor clinical outcome in triple-negative breast cancers. Cell Cycle 11(6):1108–1117
Tchou J, Conejo-Garcia J (2012) Targeting the tumor stroma as a novel treatment strategy for breast cancer: shifting from the neoplastic cell-centric to a stroma-centric paradigm. Adv Pharmacol 65:45–61
Finak G, Bertos N, Pepin F et al (2008) Stromal gene expression predicts clinical outcome in breast cancer. Nat Med 14(5):518–527
Dvorak HF (1986) Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315(26):1650–1659
Troester MA, Lee MH, Carter M et al (2009) Activation of host wound responses in breast cancer microenvironment. Clin Cancer Res 15(22):7020–7028
Dekker TJ, Balluff BD, Jones EA et al (2014) Multicenter matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI MSI) identifies proteomic differences in breast-cancer-associated stroma. J Proteome Res 13:4730–4738
Mesker WE, Junggeburt JM, Szuhai K et al (2007) The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell Oncol 29(5):387–398
de Kruijf EM, van Nes JG, van de Velde CJ et al (2011) Tumor-stroma ratio in the primary tumor is a prognostic factor in early breast cancer patients, especially in triple-negative carcinoma patients. Breast Cancer Res Treat 125(3):687–696
Dekker TJ, van de Velde CJ, van Pelt GW et al (2013) Prognostic significance of the tumor-stroma ratio: validation study in node-negative premenopausal breast cancer patients from the EORTC perioperative chemotherapy (POP) trial (10854). Breast Cancer Res Treat 139(2):371–379
Gujam FJ, Edwards J, Mohammed ZM, Going JJ, McMillan DC (2014) The relationship between the tumour stroma percentage, clinicopathological characteristics and outcome in patients with operable ductal breast cancer. Br J Cancer 111(1):157–165
Moorman AM, Vink R, Heijmans HJ, van der Palen J, Kouwenhoven EA (2012) The prognostic value of tumour-stroma ratio in triple-negative breast cancer. Eur J Surg Oncol 38(4):307–313
Blamey RW, Hornmark-Stenstam B, Ball G et al (2010) ONCOPOOL—A European database for 16,944 cases of breast cancer. Eur J Cancer 46(1):56–71
Alkushi A (2009) Validation of tissue microarray biomarker expression of breast carcinomas in Saudi women. Hematol Oncol Stem Cell Ther 2(3):394–398
Bhargava R, Lal P, Chen B (2004) Feasibility of using tissue microarrays for the assessment of HER-2 gene amplification by fluorescence in situ hybridization in breast carcinoma. Diagn Mol Pathol 13(4):213–216
Glajcar A, Kaczmarczyk K, Szpor J, Okon K (2013) Application of tissue microarrays for receptor immunohistochemistry in breast carcinoma. Folia Histochem Cytobiol 51(4):326–332
Gulbahce HE, Gamez R, Dvorak L, Forster C, Varghese L (2012) Concordance between tissue microarray and whole-section estrogen receptor expression and intratumoral heterogeneity. Appl Immunohistochem Mol Morphol 20(4):340–343
Downey CL, Simpkins SA, White J et al (2014) The prognostic significance of tumour-stroma ratio in oestrogen receptor-positive breast cancer. Br J Cancer 110(7):1744–1747
West NP, Dattani M, McShane P et al (2010) The proportion of tumour cells is an independent predictor for survival in colorectal cancer patients. Br J Cancer 102(10):1519–1523
Mesker WE, Dekker TJ, de Kruijf EM et al (2014) Comment on: the prognostic significance of tumour-stroma ratio in oestrogen receptor-positive breast cancer. Br J Cancer 112(11):1833–1834
Gucalp A, Gupta GP, Pilewskie ML, Sutton EJ, Norton L (2014) Advances in managing breast cancer: a clinical update. F1000Prime Rep 6:66
Kalluri R (2016) The biology and function of fibroblasts in cancer. Nat Rev Cancer 16:582–598
Gabrielson M, Chiesa F, Paulsson J, Strell C et al (2016) Amount of stroma is associated with mammographic density and stromal expression of oestrogen receptor in normal breast tissues. Breast Cancer Res Treat 158:253–261
Lisanti M, Tsirigos A, Pavlides S et al (2014) JNK1 stress signaling is hyper-activated in high breast density and the tumor stroma: connecting fibrosis, inflammation, and stemness for cancer prevention. Cell Cycle 13(4):580–599
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roeke, T., Sobral-Leite, M., Dekker, T.J.A. et al. The prognostic value of the tumour-stroma ratio in primary operable invasive cancer of the breast: a validation study. Breast Cancer Res Treat 166, 435–445 (2017). https://doi.org/10.1007/s10549-017-4445-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4445-8