Abstract
The rate of breast cancer screening for women of all ages in Japan is increasing. However, little is known about the biological differences between screen- and self-detected tumors. We used data from the Japanese Breast Cancer Registry (JBCR), a nationwide registry of newly diagnosed breast cancer cases in Japan, to investigate patients diagnosed between January 1, 2004 and December 31, 2011. We compared the clinicopathological features of tumors and assessed yearly trends regarding the proportion of screen-detected cases during the study period. We found that 31.8 % (65,358/205,544) of cancers were detected by screening. Asymptomatic tumors detected by screening (asymptomatic) were more likely to have favorable prognostic features than those that were self-detected (ductal carcinoma in situ [DCIS]: 19.8 versus 4.1 %, node-negative: 77.0 versus 61.6 %, and estrogen receptor-positive [ER+]: 82.0 versus 72.9 %, respectively). All these findings were statistically significant (p < .001). The proportion of breast cancers detected by screening among all cases increased from 21.7 % in 2004 to 37.1 % in 2011. During the same time period, the proportion of screen-detected DCIS increased from 41.5 to 66.0 % and that of ER+ cancers increased from 23.2 to 39.7 %. This study demonstrated that low-risk tumors, including DCIS, ER+, and lower TNM stage, account for a substantial proportion of clinical screening-detected cancers. The differences in biological characteristics between screen- and self-detected cancers may account in part for the limited efficacy of breast cancer screening programs aimed at improving breast cancer mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The goal of mammography screening is to reduce breast cancer mortality by early detection and therapy. Data from the US Preventive Services Task Force (USPSTF) indicating that breast cancer screening has reduced breast cancer mortality by 16 % have contributed to the spread of breast cancer screening around the world [1]. In the US and parts of Europe, breast cancer screening rates have reached approximately 70 % [2].
Recently, several negative reports on the efficacy of mammography screening have been published. Autier et al. compared early and late adopters of mammography screening [3] as well as specific mortality patterns in Sweden [4]; Kalager et al. assessed counties with and without screening programs in Norway [5]; Mukhtar et al. tested the effect of screening on mortality trends in England [6]; and Miller et al. analyzed 90,000 cases in Canada that were followed for 25 years [7]. All these studies showed no impact of mammography on mortality due to breast cancer. In 2009, the USPSTF recommended that women younger than 50 years need not be screened routinely, and women aged 50–74 years should have biennial rather than annual screens [8]. In 2014, the Swiss medical board reported that systematic mammography screening might prevent approximately one death attributed to breast cancer for every 1000 women screened, even though there was no evidence to suggest that overall mortality was affected. Furthermore, mammography tends to produce false positive test results and carries the risk of overdiagnosis because it detects cancers that are unlikely to shorten patients’ lives [9]. One reason to limit breast cancer screening is the improvement of adjuvant therapies [10]. The reports that recommended screening were based on studies performed over 20 years ago, and adjuvant therapy regimens have since been markedly improved. Furthermore, the screening quality at regular clinics might differ from those performed during restricted, well-designed clinical trials. With increasing rates of breast cancer screening, less aggressive breast cancers may be diagnosed, leading to overdiagnosis. The actual number of overdiagnosed cases is difficult to determine, and can only be estimated from incidence and breast cancer characteristics data. One group reported that mammography detected lower-grade and estrogen receptor (ER) positive (+) cancers upon analyzing 1983 cases [11], and that luminal A types cancers were common while epidermal growth factor 2 (HER2)+ or ER negative (−) tumors were rare in mammography-detected cancers among 1236 cases analyzed [12]. Both studies had relatively small sample sizes, and might therefore have been underpowered. To estimate the rates of overdiagnosis accurately, a larger database is required that contains data on the biological differences in breast cancer characteristics between screen- and self-detected tumors.
In Japan, mammography screening has been recommended for women 40 years of age and older since 2004. The Basic Plan to Promote Cancer Control program was developed in 2007 with the stated aim of improving the cancer screening rate to 50 % or more within 5 years [13, 14]. Breast cancer screening is not a common method of prevention in Japan, partly because the medical insurance system provided by the government differs from that in the US or Europe. Additionally, breast cancer awareness has not fully permeated the society. Nevertheless, there has been an increasing trend in breast cancer screening for women of all ages in Japan, from 24.7 % in 2007 to 34.2 % in 2013 [15].
To our knowledge, large-scale retrospective studies that analyzed breast cancer characteristics according to mode of detection in the general population of patients have not been previously published. In this study, we assessed the differences in the biological characteristics and age distributions between screen- and self-detected breast cancers using the Japan Breast Cancer Registry (JBCR) database, which includes over 200,000 newly treated breast cancers between 2004 and 2011.
Methods
Data source
This study was conducted using the JBCR database, the details of which have been previously reported by Kurebayashi et al. [16]. In short, it is a registry managed by the Registration Committee of the Japanese Breast Cancer Society, with support from the Public Health Research Foundation (Tokyo). Data on newly operated primary breast cancer patients are reported from affiliated institutes throughout Japan, which included 741 facilities in 2011, through a web-based system that collects information on more than 50 demographic and clinicopathological factors. Pathological TNM classification is registered based on the sixth edition of the Unio Internationalis Contra Cancrum staging system [17], and histological classification was according to the General Rules for Clinical and Pathological Recording of Breast Cancer [18] that was translated to the classification of Tumors of the Breast and Female Genital Organs [19].
Patient enrollment
We enrolled JBCR-listed female patients who underwent breast cancer surgery between 2004 and 2011. A nationwide project managed in cooperation with the certification board of the Japan Surgical Society [20] and an ethics review committee in the Japanese Breast Cancer Society approved the study. Patients with records of past breast cancer were excluded. For those with simultaneous bilateral tumors, we counted only one record per patient. Lastly, we excluded patients missing information on the mode of cancer detection.
Patient and tumor characteristics
Data on patient demographics and tumor characteristics, including size, lymph node involvement, presence or absence of clinical metastasis, and information on hormone receptor and HER2 expression were collected from the registry. We defined HER2 positivity as having an immunohistochemistry score of 3+ or a positive fluorescence in situ hybridization result. Hormone receptor (ER/progesterone receptor [PgR]) positivity was diagnosed if at least 1 % of nuclei in the tumor were stained on immunohistochemical tests for ER/PgR. The data on the mode of detection were also collected from the registry; the categories included “self-detection,” “screening (asymptomatic),” and “screening (symptomatic).” Screening detection included, but was not restricted to, mammography.
Statistical analysis
We analyzed the characteristics of the patients and their tumors after determining the means and standard deviations of the ages and body mass indexes (BMIs) at diagnosis, as well as the tumor characteristics. The calculations were repeated while stratifying according to the mode of detection, and the relationships between these characteristics as well as the modes of detection were subjected to Pearson’s Chi-square test for categorical variables and ANOVA for continuous variables. We further examined the yearly change in the proportions of self-detection versus screening (symptomatic or asymptomatic) in the entire cohort and in the sub-groups of ductal carcinoma in situ (DCIS), T3/T4, and ER+ cases between 2004 and 2011. Finally, we showed the age frequency distribution at diagnosis according to detection mode, overall as well as in each breast cancer subtype (DCIS, ER+, ER−, HER2+, and invasive breast cancer). All analyses were performed using SAS 9.3 (SAS Institute, Cary, NC, USA).
Results
Study population
We enrolled 205,554 patients in the study cohort (Fig. 1). The patients had a mean age of 58.0 ± 13.1 years and BMI of 22.8 ± 3.8 (Table 1). As for tumor characteristics, 9.8 % (20,148/205,544) of cases were DCIS, 48.4 % were T1, and 7.6 % were T3 and above. Furthermore, 66.0 % were node-negative, while 75.6, 62.7, and 13.3 % of tumors were ER+, PgR+, and HER2+, respectively.
Breast cancer characteristics by the mode of detection
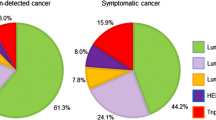
Distributions of the three modes of detection with respect to patient and tumor characteristics, including TNM stage, ER, PgR, and HER2 status, are shown in Table 2. The number of self-detected cases was 140,186, whereas 12,202 cases were screen-detected after exhibiting symptoms, while a further 53,156 were detected by screening (asymptomatic). Hence, 31.8 % (65,358/205,577) of cases were detected by breast cancer screening. Patients with self-detected tumors were on average significantly older than those with screen-detected tumors, whether symptomatic or asymptomatic. The distribution of TNM stage differed according to the mode of detection: among the asymptomatic cases detected by screening (asymptomatic), 19.8 % were DCIS, whereas only 4.1 % of the self-detected cases were DCIS. The proportion of T3 or T4 tumors was highest in the self-detected cases, at 9.8 %; the rate was 6.0 % for symptomatic screen-detected cases; and 2.1 % for screen-detected asymptomatic cases. Self-detected cases were also more likely to have node-positive tumors (33.1 %) compared to either symptomatic screen-detected cases (27.1 %) or asymptomatic screen-detected cases (15.6 %). Similarly, the prevalence of M-positives tumors was highest in self-detected cases. The distribution of breast cancer receptor status differed by the mode of detection: a higher prevalence of both ER+ and PgR+ breast cancers, which have relatively less aggressive behaviors, were observed among cases screen-detected than among those that were self-detected. Conversely, HER2+ and ER− cancers were more prevalent in self-detected cases.
Annual changes in the proportions of cases according to mode of detection
Next, we assessed the change in the proportion of breast cancers detected by each mode between the years 2004 and 2011. The proportion of tumors detected by screening in asymptomatic patients was only 16.0 % in 2004. Seven years later, this proportion reached 30.4 %, i.e., it almost doubled. On the other hand, the proportion of self-detected breast cancer cases decreased from 78.3 % in 2004 to 62.9 % in 2011 (Fig. 2a). In the DCIS subgroup, the proportion of cases detected by screening (symptomatic and asymptomatic) increased from 41.5 % in 2004 to 66.0 % in 2011 (Fig. 2b). Only 9.3 % of T3/T4 cancer cases were detected by screening in 2004, and while the proportion grew over time, it remained low at 13.8 % in 2011 (Fig. 2c). The trend of cases being increasingly detected clinically also extended to the ER+ subgroup of patients, where detection grew from 23.2 % in 2004 to 39.7 % in 2011 (Fig. 2d). To exclude the effect of DCIS cases, we assessed the yearly change for invasive cancers only. The trend for screen-detected invasive ER+ cases similarly grew from 21.8 % in 2004 to 35.8 % in 2011 (data not shown).
Age frequency distribution at diagnosis by mode of detection and breast cancer subtypes
Finally, we assessed age frequency distribution at diagnosis by mode of detection and breast cancer subtype. In all breast cancer cases as well as invasive breast cancers, all three detection modes had similar bimodal distributions (Fig. 3a, supplementary Fig. 1). Cases with DCIS and ER+, which have relatively favorable clinical courses, had bimodal distributions with the higher peak at around 40 years of age (Fig. 3b, c). On the other hand, cases with ER- and HER2+, which have relatively poor prognoses, had one-peak at around 60 years old (Fig. 3d; supplementary Fig. 2), with no differences according to modes of detection.
Density plots for age frequency distribution at diagnosis, by detection mode density plots show the age frequency distribution at diagnosis, according to detection mode, in a all breast cancers, b DCIS, c ER+ tumors, and d ER− tumors between 2004 and 2011. DCIS ductal carcinoma in situ; ER estrogen receptor
Discussion
Our study exposed biological differences between breast tumors discovered screen versus those that are self-detected. We found that screen-detected cancers reported in the JBCR database were associated with less aggressive characteristics (e.g., DCIS and ER+) that produced a bimodal age distribution pattern. All breast cancers registered in the JBCR between 2004 and 2011, representing approximately 70 % of newly diagnosed breast cancer patients in Japan, were analyzed. As patients were systematically recorded in the JBCR database, selection bias was presumably reduced and false positive results minimized.
We showed significant variations in breast cancer receptor statuses between screen and self-detected cases; more prognostically favorable lesions (ER+/PgR+/HER2−) were observed in screen-detected cancers. Moreover, lower TNM stages and DCIS were more frequently discovered screen-detected tumors. Previous papers have similarly shown favorable trends in clinically detected cancers [11, 12]. The propensity toward DCIS was notable in our study, as we showed that the DCIS rate in screen-detected cancers was almost 20 % of all revealed breast cancers. Of all DCIS cases, those that were screen-detected represented almost 40 % in 2004, gradually increasing to over 60 % in 2011. A previous study reported that the incidence of DCIS has steadily increased in all countries and among all ages [21]. As mammography screening becomes more widespread, the larger numbers of DCIS patients are likely to be discovered. In 2004, mammotome biopsy using radiography or ultrasound was adopted by the Japanese National Health Insurance program. Mammotome biopsy is an accurate technique for the sampling and diagnosis of breast cancers exhibiting only microcalcification [22]. This may be another reason for the increasing numbers of DCIS during our study period in Japan [22]. DCIS have an extremely favorable prognosis after surgery [23–25], although the natural course of DCIS remains unclear. Small retrospective studies showed that some cases may progress to invasive breast cancers in the absence of any therapeutic interventions, whereas others do not. Only 32 % of DCIS tumors observed over a 30-year follow-up period progressed to invasive cancers, and 11 % of such tumors progressed to invasiveness over a 17-year follow-up period, in previously reported studies [26, 27]. In other words, the majority of DCIS cancers are likely to remain uninvasive for a significant period of time. The increasing numbers of detected DCIS lesions may lead to overtreatment, which is one of the limitations regarding the efficacy of breast cancer screening, especially as there is no way to determine which DCIS will progress to invasive cancer. The rate of DCIS as a ratio of overall cancers detected by mammography is highest in women aged 40–49 years [28]. Our data also revealed a bimodal DCIS population, with the higher peak at approximately 40 years of age and the lower at 60 years (Fig. 3b), indicating possible heterogeneous characteristics (e.g., ER±, comedo vs. non-comedo type, or high vs. low grade) within DCIS; however, no further information was available in the JBCR. Bimodal distribution patterns can result naturally from such variable characteristics due to the existence of two distinct sub-groups of patients [29]; understanding these heterogeneities may produce novel treatment strategies for DCIS in the future. One ongoing clinical trial (the LORIS trial) that aims to determine whether patients with low or intermediate grade DCIS can forgo surgery may provide important insights regarding DCIS treatment in clinical practice [30].
Another interesting finding was the bimodal breast cancer population by age overall, including ER+ and invasive cases. Bimodal distribution for breast cancers overall have been reported in Europe and the US [31–33], Africa [34], Taiwan [35], and New Zealand [36]. The classically recognized inflection point in age-specific breast cancer incidence that occurs around menopause, known as “Clemmesen’s Hook [37, 38] ,” may reflect the superimposition of two different rate curves that represent extremely diverse clinicopathological and/or etiological characteristics, including ER± cancers or sporadic/familial cancers. Additionally, low-risk breast cancers, including ER+ tumors and DCIS, exhibit similar bimodal distribution patterns, suggesting two distinct biological characteristics and etiologies for early versus late onset tumors. The bimodal distribution pattern for ER+ cancers may suggest the influence of other composite breast cancer subtypes, including luminal A/B or HER2± tumors, within ER+ cases. On the other hand, high-risk cancers such as ER− or HER2+ cases showed a unimodal age frequency distribution peeking at age 60 years, suggesting that the etiologies of these particular tumors may be independent of menopause. Our results showed a relatively late onset for high-risk cancers that are ER− and HER2+. However, some previous studies showed that high-risk breast cancers tend to develop in younger rather than in older patients [39–41]. The relatively higher frequency of breast cancers positive for BRCA1/2 mutations in Japan (27.2 %) may result in different age distributions compared to other countries [42].
The USPSTF suggested that undergoing regular, biennial screening mammograms before the age of 50 years should be an individual decision (grade C recommendation), taking into account a patient’s context, as well as the benefits and potential pitfalls of such screenings [43]. Following USPSTF recommendations, the Japan Association of Breast Cancer Screening reassessed the efficacy of screening mammography, taking into consideration both benefits and drawbacks, in 2010 [44]. The Association collected mammography screening data from 144,848 participants from five prefectures, and concluded that the disadvantages of screening mammograms for Japanese women were less than those for American women because of lower false positive rates, additional imaging, and biopsy methods (including biopsy invasiveness) [44]. Our data showed that there was a peak in the frequency of low-risk cancers at approximately 40 years of age, and that these patterns were different from those found in the US and Europe [45]. That these distinct patterns were observed in Japan may mean that breast cancer screening advantages may vary compared to those in the US and Europe.
We acknowledge several important limitations. First, this study was retrospective, incurring the possibility of selection bias and precluding the determination of causal relationships. However, JBCR data represent approximately 70 % of patients diagnosed with breast cancer in Japan [16]; therefore, it is unlikely that any bias would substantially affect our findings. Second, our study was subject to limitations associated with analyzing registry data, such as the lack of standardization of histopathologic diagnoses and receptor status testing. Finally, breast cancer screening in Japan does not necessarily entail mammography, and few participants received only clinical breast examinations without mammography. However, the strength of our study is that it is based on over 200,000 patients in a “real-world” setting.
In conclusion, our study demonstrated that low-risk tumors, including DCIS, ER+, and lower TNM stage lesions, account for a substantial proportion of clinical screen-detected cancers. These different biological characteristics between screen-detected and self-detected cancers may account in part for the reported limitations of breast cancer screening programs aimed at reducing breast cancer mortality. Indeed, there are likely to be some disadvantages of breast cancer screening in patients with slow-growing and less-aggressive cancers. To establish an optimal breast cancer screening system would require a larger study with a longer follow-up period to identify those sub-groups with higher risks, and also to have a sufficient number of subjects in each subgroup to effectively compare outcomes with or without breast cancer screening. Such data are unlikely to be generated in the near feature. In the meantime, since the potential life-saving benefit of early detection cannot be discounted, the safest approach may be to promote breast cancer screening practices in Japan, where rates of such screenings are extremely low because of current guidelines. At the same time, clear and unbiased information should be provided as part of public health initiatives. Future studies will require the creation of national and international cooperative networks to ensure consistency and reproducibility of distinct breast cancer characteristics between screen-detected and self-detected cancers.
References
Humphrey LL, Helfand M, Chan BK, Woolf SH (2002) Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 137(5 Part 1):347–360
Saika K, Sobue T (2011) Time trends in breast cancer screening rates in the OECD countries. Jpn J Clin Oncol 41(4):591–592. doi:10.1093/jjco/hyr044
Autier P, Boniol M, Gavin A, Vatten LJ (2011) Breast cancer mortality in neighbouring European countries with different levels of screening but similar access to treatment: trend analysis of WHO mortality database. BMJ 343:d4411
Autier P, Koechlin A, Smans M, Vatten L, Boniol M (2012) Mammography screening and breast cancer mortality in Sweden. J Natl Cancer Inst 104(14):1080–1093. doi:10.1093/jnci/djs272
Kalager M, Zelen M, Langmark F, Adami HO (2010) Effect of screening mammography on breast-cancer mortality in Norway. N Engl J Med 363(13):1203–1210. doi:10.1056/NEJMoa1000727
Mukhtar TK, Yeates DR, Goldacre MJ (2013) Breast cancer mortality trends in England and the assessment of the effectiveness of mammography screening: population-based study. J R Soc Med 106(6):234–242. doi:10.1177/0141076813486779
Miller AB, Wall C, Baines CJ, Sun P, To T, Narod SA (2014) Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ 348:g366
Woolf SH (2010) The 2009 breast cancer screening recommendations of the US Preventive Services Task Force. JAMA 303(2):162–163. doi:10.1001/jama.2009.1989
Biller-Andorno N, Juni P (2014) Abolishing mammography screening programs? A view from the Swiss Medical Board. N Engl J Med. doi:10.1056/NEJMp1401875
Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717. doi:10.1016/S0140-6736(05)66544-0
Joensuu H, Lehtimaki T, Holli K, Elomaa L, Turpeenniemi-Hujanen T, Kataja V, Anttila A, Lundin M, Isola J, Lundin J (2004) Risk for distant recurrence of breast cancer detected by mammography screening or other methods. JAMA 292(9):1064–1073. doi:10.1001/jama.292.9.1064
Sihto H, Lundin J, Lehtimaki T, Sarlomo-Rikala M, Butzow R, Holli K, Sailas L, Kataja V, Lundin M, Turpeenniemi-Hujanen T, Isola J, Heikkila P, Joensuu H (2008) Molecular subtypes of breast cancers detected in mammography screening and outside of screening. Clin Cancer Res 14(13):4103–4110. doi:10.1158/1078-0432.CCR-07-5003
Ministry of Health, Labor and Welfare of Japan (2007). http://www.mhlw.go.jp/shingi/2007/06/s0615-1.html. Accessed Aug 2015
Tsunematsu M, Kawasaki H, Masuoka Y, Kakehashi M (2013) Factors affecting breast cancer screening behavior in Japan—assessment using the health belief model and conjoint analysis. Asian Pac J Cancer Prev 14(10):6041–6048
Ministry of Health, Labor and Welfare of Japan. http://www.mhlw.go.jp/toukei/saikin/hw/k-tyosa/k-tyosa13/index.html. Accessed Aug 2015
Kurebayashi J, Miyoshi Y, Ishikawa T, Saji S, Sugie T, Suzuki T, Takahashi S, Nozaki M, Yamashita H, Tokuda Y, Nakamura S (2015) Clinicopathological characteristics of breast cancer and trends in the management of breast cancer patients in Japan: based on the Breast Cancer Registry of the Japanese Breast Cancer Society between 2004 and 2011. Breast Cancer 22(3):235–244. doi:10.1007/s12282-015-0599-6
Greene F (2002) TNM classification of malignant tumours, 6th edn. Wiley, New York, pp 131–141
The Japanese Breast Cancer Society (ed) (2012) General rules for clinical and pathological recording of breast cancer, vol 17. Kanehara Shuppan, Tokyo
Lakhani S, Ellis I, Schnitt S, Tan P, van de Vijver M (2012) WHO classification of tumours of the breast, 4th edn. IARC Press, Lyon
Miyata H, Gotoh M, Hashimoto H, Motomura N, Murakami A, Tomotaki A, Hirahara N, Ono M, Ko C, Iwanaka T (2014) Challenges and prospects of a clinical database linked to the board certification system. Surg Today 44(11):1991–1999. doi:10.1007/s00595-013-0802-3
Recht A, Rutgers EJ, Fentiman IS, Kurtz JM, Mansel RE, Sloane JP (1998) The fourth EORTC DCIS consensus meeting (Chateau Marquette, Heemskerk, The Netherlands, 23–24 January 1998)—conference report. Eur J Cancer 34(11):1664–1669
Cangiarella J, Waisman J, Symmans WF, Gross J, Cohen JM, Wu H, Axelrod D (2001) Mammotome core biopsy for mammary microcalcification: analysis of 160 biopsies from 142 women with surgical and radiologic followup. Cancer 91(1):173–177
Kinoshita T, Fukui N, Anan K, Iwamoto T, Niikura N, Kawai M, Hayashi N, Tsugawa K, Aogi K, Ishida T, Masuoka H, Masuda S, Iijima K, Nakamura S, Tokuda Y (2015) Comprehensive prognostic report of the Japanese Breast Cancer Society Registry in 2004. Breast Cancer. doi:10.1007/s12282-015-0644-5
Anan K, Fukui N, Kinoshita T, Iwamoto T, Niikura N, Kawai M, Hayashi N, Tsugawa K, Aogi K, Ishida T, Masuoka H, Masuda S, Iijima K, Nakamura S, Tokuda Y (2015) Comprehensive prognostic report of the Japanese Breast Cancer Society Registry in 2005. Breast Cancer. doi:10.1007/s12282-015-0645-4
Iwamoto T, Fukui N, Kinoshita T, Anan K, Niikura N, Kawai M, Hayashi N, Tsugawa K, Aogi K, Ishida T, Masuoka H, Masuda S, Iijima K, Nakamura S, Tokuda Y (2015) Comprehensive prognostic report of the Japanese Breast Cancer Society Registry in 2006. Breast Cancer. doi:10.1007/s12282-015-0646-3
Page DL, Dupont WD, Rogers LW, Landenberger M (1982) Intraductal carcinoma of the breast: follow-up after biopsy only. Cancer 49(4):751–758
Eusebi V, Foschini MP, Cook MG, Berrino F, Azzopardi JG (1989) Long-term follow-up of in situ carcinoma of the breast with special emphasis on clinging carcinoma. Semin Diagn Pathol 6(2):165–173
Ernster VL, Ballard-Barbash R, Barlow WE, Zheng Y, Weaver DL, Cutter G, Yankaskas BC, Rosenberg R, Carney PA, Kerlikowske K, Taplin SH, Urban N, Geller BM (2002) Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst 94(20):1546–1554
Wang J, Wen S, Symmans WF, Pusztai L, Coombes KR (2009) The bimodality index: a criterion for discovering and ranking bimodal signatures from cancer gene expression profiling data. Cancer Inform 7:199–216
LORIS trial. http://shore-c.sussex.ac.uk/loris.htm. Accessed Aug 2015
de Waard F, de Laive JWJ (1960) On the bimodal age distribution of mammary carcinoma. Br J Cancer 14:437–448
Anderson WF, Pfeiffer RM, Dores GM, Sherman ME (2006) Comparison of age distribution patterns for different histopathologic types of breast carcinoma. Cancer Epidemiol Biomark Prev 15(10):1899–1905. doi:10.1158/1055-9965.EPI-06-0191
Sant M, Gatta G, Micheli A, Verdecchia A, Capocaccia R, Crosignani P, Berrino F (1991) Survival and age at diagnosis of breast cancer in a population-based cancer registry. Eur J Cancer 27(8):981–984
Muguti GI (1993) Experience with breast cancer in Zimbabwe. J R Coll Surg Edinb 38(2):75–78
Chie WC, Chen CF, Lee WC, Chen CJ, Lin RS (1995) Age-period-cohort analysis of breast cancer mortality. Anticancer Res 15(2):511–515
Newman PD, Mason BH, Holdaway IM, Kay RG, Arthur JF, Hitchcock GC (1992) Incidence and clinical features of breast cancer in the Auckland region. N Z Med J 105(931):117–120
Yasui Y, Potter JD (1999) The shape of age-incidence curves of female breast cancer by hormone-receptor status. Cancer Causes Control 10(5):431–437
Tarone RE, Chu KC (2002) The greater impact of menopause on ER − than ER + breast cancer incidence: a possible explanation (United States). Cancer Causes Control 13(1):7–14
Anderson WF, Chatterjee N, Ershler WB, Brawley OW (2002) Estrogen receptor breast cancer phenotypes in the surveillance, epidemiology, and end results database. Breast Cancer Res Treat 76(1):27–36
Anderson WF, Rosenberg PS, Menashe I, Mitani A, Pfeiffer RM (2008) Age-related crossover in breast cancer incidence rates between black and white ethnic groups. J Natl Cancer Inst 100(24):1804–1814. doi:10.1093/jnci/djn411
Anderson WF, Rosenberg PS, Prat A, Perou CM, Sherman ME (2014) How many etiological subtypes of breast cancer: two, three, four, or more? J Natl Cancer Inst. doi:10.1093/jnci/dju165
Sugano K, Nakamura S, Ando J, Takayama S, Kamata H, Sekiguchi I, Ubukata M, Kodama T, Arai M, Kasumi F, Hirai Y, Ikeda T, Jinno H, Kitajima M, Aoki D, Hirasawa A, Takeda Y, Yazaki K, Fukutomi T, Kinoshita T, Tsunematsu R, Yoshida T, Izumi M, Umezawa S, Yagata H, Komatsu H, Arimori N, Matoba N, Gondo N, Yokoyama S, Miki Y (2008) Cross-sectional analysis of germline BRCA1 and BRCA2 mutations in Japanese patients suspected to have hereditary breast/ovarian cancer. Cancer Sci 99(10):1967–1976. doi:10.1111/j.1349-7006.2008.00944.x
Force USPST (2009) Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 151:716–726
Kasahara Y, Kawai M, Tsuji I, Tohno E, Yokoe T, Irahara M, Tangoku A, Ohuchi N (2013) Harms of screening mammography for breast cancer in Japanese women. Breast Cancer 20(4):310–315. doi:10.1007/s12282-012-0333-6
Bray F, McCarron P, Parkin DM (2004) The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res 6(6):229–239. doi:10.1186/bcr932
Acknowledgments
HK, HM, and AT are affiliated with the Department of Healthcare Quality Assessment at the University of Tokyo, and the department is endowed by Johnson & Johnson K.K., Nipro Corporation, Teijin Pharma Ltd., Kaketsuken K.K., St. Jude Medical Japan Co., Ltd., Novartis Pharma K.K., Taiho Pharmaceutical Co., Ltd., W. L. Gore & Associates, Co., Ltd., Olympus Corporation, and Chugai Pharmaceutical Co., Ltd. This work was supported partly by JSPS KAKENHI Grant Numbers 15H04796.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2016_3770_MOESM1_ESM.pptx
Density plots of age frequency distribution at diagnosis, by detection mode, for invasive cancers. Supplementary material 1 (PPTX 87 kb)
10549_2016_3770_MOESM2_ESM.pptx
Density plots of age frequency distribution at diagnosis, by detection mode, for HER2 + cancers. HER2: Human epidermal growth factor receptor 2. Supplementary material 2 (PPTX 86 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Iwamoto, T., Kumamaru, H., Miyata, H. et al. Distinct breast cancer characteristics between screen- and self-detected breast cancers recorded in the Japanese Breast Cancer Registry. Breast Cancer Res Treat 156, 485–494 (2016). https://doi.org/10.1007/s10549-016-3770-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3770-7