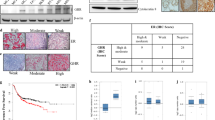
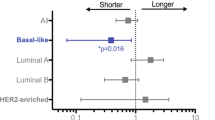
Abstract
The purpose of this study was to discover novel nuclear receptor targets in triple-negative breast cancer. Expression microarray, Western blot, qRT-PCR analyses, MTT growth assay, soft agar anchorage-independent growth assay, TRE reporter transactivation assay, and statistical analysis were performed in this study. We performed microarray analysis using 227 triple-negative breast tumors, and clustered the tumors into five groups according to their nuclear receptor expression. Thyroid hormone receptor beta (TRβ) was one of the most differentially expressed nuclear receptors in group 5 compared to other groups. TRβ low expressing patients were associated with poor outcome. We evaluated the role of TRβ in triple-negative breast cancer cell lines representing group 5 tumors. Knockdown of TRβ increased soft agar colony and reduced sensitivity to docetaxel and doxorubicin treatment. Docetaxel or doxorubicin long-term cultured cell lines also expressed decreased TRβ protein. Microarray analysis revealed cAMP/PKA signaling was the only KEGG pathways upregulated in TRβ knockdown cells. Inhibitors of cAMP or PKA, in combination with doxorubicin further enhanced cell apoptosis and restored sensitivity to chemotherapy. TRβ-specific agonists enhanced TRβ expression, and further sensitized cells to both docetaxel and doxorubicin. Sensitization was mediated by increased apoptosis with elevated cleaved PARP and caspase 3. TRβ represents a novel nuclear receptor target in triple-negative breast cancer; low TRβ levels were associated with enhanced resistance to both docetaxel and doxorubicin treatment. TRβ-specific agonists enhance chemosensitivity to these two agents. Mechanistically enhanced cAMP/PKA signaling was associated with TRβ’s effects on response to chemotherapy.




Similar content being viewed by others
Abbreviations
- AR:
-
Androgen receptor
- CIS or C:
-
Cisplatin
- DOC or T:
-
Docetaxel
- DOX or D:
-
Doxorubicin
- ERα:
-
Estrogen receptor alpha
- EV:
-
Empty vector
- KD:
-
Knockdown
- LAR:
-
Luminal AR
- NRs:
-
Nuclear receptors
- PAM:
-
Prediction analysis of microarrays
- TRβ:
-
Thyroid hormone receptor beta
- TNBC:
-
Triple-negative breast cancer
References
Cuzick J, Sestak I, Bonanni B, Costantino JP, Cummings S, DeCensi A, Dowsett M, Forbes JF, Ford L, LaCroix AZ, Mershon J, Mitlak BH, Powles T, Veronesi U, Vogel V, Wickerham DL, Group SCOBCO (2013) Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet 381(9880):1827–1834. doi:10.1016/S0140-6736(13)60140-3
Early Breast Cancer Trialists’ Collaborative G, Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378(9793):771–784. doi:10.1016/S0140-6736(11)60993-8
Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, Zambetti M, Vazquez F, Byakhow M, Lichinitser M, Climent MA, Ciruelos E, Ojeda B, Mansutti M, Bozhok A, Baronio R, Feyereislova A, Barton C, Valagussa P, Baselga J (2010) Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 375(9712):377–384. doi:10.1016/S0140-6736(09)61964-4
Foulkes WD, Smith IE, Reis-Filho JS (2010) Triple-negative breast cancer. N Engl J Med 363(20):1938–1948. doi:10.1056/NEJMra1001389
Turner N, Moretti E, Siclari O, Migliaccio I, Santarpia L, D’Incalci M, Piccolo S, Veronesi A, Zambelli A, Del Sal G, Di Leo A (2013) Targeting triple negative breast cancer: is p53 the answer? Cancer Treat Rev 39(5):541–550. doi:10.1016/j.ctrv.2012.12.001
Buzdar AU, Singletary SE, Theriault RL, Booser DJ, Valero V, Ibrahim N, Smith TL, Asmar L, Frye D, Manuel N, Kau SW, McNeese M, Strom E, Hunt K, Ames F, Hortobagyi GN (1999) Prospective evaluation of paclitaxel versus combination chemotherapy with fluorouracil, doxorubicin, and cyclophosphamide as neoadjuvant therapy in patients with operable breast cancer. J Clin Oncol 17(11):3412–3417
Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26(8):1275–1281. doi:10.1200/JCO.2007.14.4147
von Minckwitz G, Martin M (2012) Neoadjuvant treatments for triple-negative breast cancer (TNBC). Ann Oncol 23(6):vi35–vi39. doi:10.1093/annonc/mds193
Mayer IA, Abramson VG, Lehmann BD, Pietenpol JA (2014) New strategies for triple-negative breast cancer–deciphering the heterogeneity. Clin Cancer Res 20(4):782–790. doi:10.1158/1078-0432.CCR-13-0583
de Hoon JP, Veeck J, Vriens BE, Calon TG, van Engeland M, Tjan-Heijnen VC (1825) Taxane resistance in breast cancer: a closed HER2 circuit? Biochim Biophys Acta 2:197–206. doi:10.1016/j.bbcan.2012.01.001
Murray S, Briasoulis E, Linardou H, Bafaloukos D, Papadimitriou C (2012) Taxane resistance in breast cancer: mechanisms, predictive biomarkers and circumvention strategies. Cancer Treat Rev 38(7):890–903. doi:10.1016/j.ctrv.2012.02.011
Chan S, Friedrichs K, Noel D, Pinter T, Van Belle S, Vorobiof D, Duarte R, Gil M, Bodrogi I, Murray E, Yelle L, von Minckwitz G, Korec S, Simmonds P, Buzzi F, Gonzalez-Mancha R, Richardson G, Walpole E, Ronzoni M, Murawsky M, Alakl M, Riva A, Crown J, Study G (1999) Prospective randomized trial of docetaxel versus doxorubicin in patients with metastatic breast cancer. J Clin Oncol 17(8):2341–2354
Evans TR, Yellowlees A, Foster E, Earl H, Cameron DA, Hutcheon AW, Coleman RE, Perren T, Gallagher CJ, Quigley M, Crown J, Jones AL, Highley M, Leonard RC, Mansi JL (2005) Phase III randomized trial of doxorubicin and docetaxel versus doxorubicin and cyclophosphamide as primary medical therapy in women with breast cancer: an anglo-celtic cooperative oncology group study. J Clin Oncol 23(13):2988–2995. doi:10.1200/JCO.2005.06.156
Nabholtz JM, Senn HJ, Bezwoda WR, Melnychuk D, Deschenes L, Douma J, Vandenberg TA, Rapoport B, Rosso R, Trillet-Lenoir V, Drbal J, Molino A, Nortier JW, Richel DJ, Nagykalnai T, Siedlecki P, Wilking N, Genot JY, Hupperets PS, Pannuti F, Skarlos D, Tomiak EM, Murawsky M, Alakl M, Aapro M et al (1999) Prospective randomized trial of docetaxel versus mitomycin plus vinblastine in patients with metastatic breast cancer progressing despite previous anthracycline-containing chemotherapy. 304 study group. J Clin Oncol 17(5):1413–1424
Jassem J, Pienkowski T, Pluzanska A, Jelic S, Gorbunova V, Mrsic-Krmpotic Z, Berzins J, Nagykalnai T, Wigler N, Renard J, Munier S, Weil C, Central Eastern E, Israel Pacitaxel Breast Cancer Study G (2001) Doxorubicin and paclitaxel versus fluorouracil, doxorubicin, and cyclophosphamide as first-line therapy for women with metastatic breast cancer: final results of a randomized phase III multicenter trial. J Clin Oncol 19(6):1707–1715
Prat A, Lluch A, Albanell J, Barry WT, Fan C, Chacon JI, Parker JS, Calvo L, Plazaola A, Arcusa A, Segui-Palmer MA, Burgues O, Ribelles N, Rodriguez-Lescure A, Guerrero A, Ruiz-Borrego M, Munarriz B, Lopez JA, Adamo B, Cheang MC, Li Y, Hu Z, Gulley ML, Vidal MJ, Pitcher BN, Liu MC, Citron ML, Ellis MJ, Mardis E, Vickery T, Hudis CA, Winer EP, Carey LA, Caballero R, Carrasco E, Martin M, Perou CM, Alba E (2014) Predicting response and survival in chemotherapy-treated triple-negative breast cancer. Br J Cancer 111(8):1532–1541. doi:10.1038/bjc.2014.444
Criscitiello C, Azim HA Jr, Schouten PC, Linn SC, Sotiriou C (2012) Understanding the biology of triple-negative breast cancer. Ann Oncol 23(6):vi13–vi18. doi:10.1093/annonc/mds188
Iwamoto T, Bianchini G, Booser D, Qi Y, Coutant C, Shiang CY, Santarpia L, Matsuoka J, Hortobagyi GN, Symmans WF, Holmes FA, O’Shaughnessy J, Hellerstedt B, Pippen J, Andre F, Simon R, Pusztai L (2011) Gene pathways associated with prognosis and chemotherapy sensitivity in molecular subtypes of breast cancer. J Natl Cancer Inst 103(3):264–272. doi:10.1093/jnci/djq524
Doane AS, Danso M, Lal P, Donaton M, Zhang L, Hudis C, Gerald WL (2006) An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene 25(28):3994–4008. doi:10.1038/sj.onc.1209415
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121(7):2750–2767. doi:10.1172/JCI45014
Gucalp A, Tolaney S, Isakoff SJ, Ingle JN, Liu MC, Carey LA, Blackwell KL, Rugo H, Nabell L, Forero-Torres A, Stearns V, Doane AS, Danso M, Moynahan ME, Momen LF, Gonzalez JM, Akhtar A, Giri D, Patil S, Feigin KN, Hudis CA, Traina TA (2013) Phase II trial of bicalutamide in patients with androgen receptor positive. Hormone receptor negative metastatic breast cancer. Clin Cancer Res. doi:10.1158/1078-0432.CCR-12-3327
Muscat GE, Eriksson NA, Byth K, Loi S, Graham D, Jindal S, Davis MJ, Clyne C, Funder JW, Simpson ER, Ragan MA, Kuczek E, Fuller PJ, Tilley WD, Leedman PJ, Clarke CL (2013) Research resource: nuclear receptors as transcriptome: discriminant and prognostic value in breast cancer. Mol Endocrinol 27(2):350–365. doi:10.1210/me.2012-1265
Martinez-Iglesias O, Garcia-Silva S, Tenbaum SP, Regadera J, Larcher F, Paramio JM, Vennstrom B, Aranda A (2009) Thyroid hormone receptor beta1 acts as a potent suppressor of tumor invasiveness and metastasis. Cancer Res 69(2):501–509. doi:10.1158/0008-5472.CAN-08-2198
Guigon CJ, Cheng SY (2009) Novel non-genomic signaling of thyroid hormone receptors in thyroid carcinogenesis. Mol Cell Endocrinol 308(1–2):63–69. doi:10.1016/j.mce.2009.01.007
Brent GA (2012) Mechanisms of thyroid hormone action. J Clin Invest 122(9):3035–3043. doi:10.1172/JCI60047
Cheng SY, Leonard JL, Davis PJ (2010) Molecular aspects of thyroid hormone actions. Endocr Rev 31(2):139–170. doi:10.1210/er.2009-0007
Suhane S, Ramanujan VK (2011) Thyroid hormone differentially modulates Warburg phenotype in breast cancer cells. Biochem Biophys Res Commun 414(1):73–78. doi:10.1016/j.bbrc.2011.09.024
Trost SU, Swanson E, Gloss B, Wang-Iverson DB, Zhang H, Volodarsky T, Grover GJ, Baxter JD, Chiellini G, Scanlan TS, Dillmann WH (2000) The thyroid hormone receptor-beta-selective agonist GC-1 differentially affects plasma lipids and cardiac activity. Endocrinology 141(9):3057–3064
Grover GJ, Mellstrom K, Ye L, Malm J, Li YL, Bladh LG, Sleph PG, Smith MA, George R, Vennstrom B, Mookhtiar K, Horvath R, Speelman J, Egan D, Baxter JD (2003) Selective thyroid hormone receptor-beta activation: a strategy for reduction of weight, cholesterol, and lipoprotein (a) with reduced cardiovascular liability. Proc Natl Acad Sci USA 100(17):10067–10072. doi:10.1073/pnas.1633737100
Johansson L, Rudling M, Scanlan TS, Lundasen T, Webb P, Baxter J, Angelin B, Parini P (2005) Selective thyroid receptor modulation by GC-1 reduces serum lipids and stimulates steps of reverse cholesterol transport in euthyroid mice. Proc Natl Acad Sci USA 102(29):10297–10302. doi:10.1073/pnas.0504379102
Burstein MD, Tsimelzon A, Poage GM, Covington KR, Contreras A, Fuqua S, Savage M, Osborne CK, Hilsenbeck SG, Chang JC, Mills GB, Lau CC, Brown PH (2014) Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. doi:10.1158/1078-0432.CCR-14-0432
Bosch A, Eroles P, Zaragoza R, Vina JR, Lluch A (2010) Triple-negative breast cancer: molecular features, pathogenesis, treatment and current lines of research. Cancer Treat Rev 36(3):206–215. doi:10.1016/j.ctrv.2009.12.002
Tibshirani R, Hastie T, Narasimhan B, Chu G (2002) Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci USA 99(10):6567–6572. doi:10.1073/pnas.082099299
Sabatier R, Finetti P, Cervera N, Lambaudie E, Esterni B, Mamessier E, Tallet A, Chabannon C, Extra JM, Jacquemier J, Viens P, Birnbaum D, Bertucci F (2011) A gene expression signature identifies two prognostic subgroups of basal breast cancer. Breast Cancer Res Treat 126(2):407–420. doi:10.1007/s10549-010-0897-9
Chinnaiyan AM (1999) The apoptosome: heart and soul of the cell death machine. Neoplasia 1(1):5–15
Boulares AH, Yakovlev AG, Ivanova V, Stoica BA, Wang G, Iyer S, Smulson M (1999) Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem 274(33):22932–22940
da Huang W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4(1):44–57. doi:10.1038/nprot.2008.211
Makin G, Hickman JA (2000) Apoptosis and cancer chemotherapy. Cell Tissue Res 301(1):143–152
Abel ED, Boers ME, Pazos-Moura C, Moura E, Kaulbach H, Zakaria M, Lowell B, Radovick S, Liberman MC, Wondisford F (1999) Divergent roles for thyroid hormone receptor beta isoforms in the endocrine axis and auditory system. J Clin Invest 104(3):291–300. doi:10.1172/JCI6397
Huang J, Jin L, Ji G, Xing L, Xu C, Xiong X, Li H, Wu K, Ren G, Kong L (2013) Implication from thyroid function decreasing during chemotherapy in breast cancer patients: chemosensitization role of triiodothyronine. BMC Cancer 13:334. doi:10.1186/1471-2407-13-334
Ladenson PW, Kristensen JD, Ridgway EC, Olsson AG, Carlsson B, Klein I, Baxter JD, Angelin B (2010) Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. N Engl J Med 362(10):906–916. doi:10.1056/NEJMoa0905633
Safa M, Kazemi A, Zand H, Azarkeivan A, Zaker F, Hayat P (2010) Inhibitory role of cAMP on doxorubicin-induced apoptosis in pre-B ALL cells through dephosphorylation of p53 serine residues. Apoptosis 15(2):196–203. doi:10.1007/s10495-009-0417-8
Acknowledgments
The authors would like to thank Dr. Paul Webb from Houston Methodist Research Institute for kindly providing us the TRβ agonists GC-1 and KB-141. We would like to thank Dr. Meng Gao for generating the heat map of Fig. 1a. This work was supported by NIH/NCI R01-CA72038, CPRIT RP120732-P2, and Susan G Komen for the Cure PG12221410.
Conflict of interest
Authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2015_3354_MOESM1_ESM.pptx
Sup. Figure 1.Hierarchical clustering was performed using 41 NR’s to classify227 ER-negative tumors. Contributing receptors were scored using prediction analysis of microarrays [33] across clustered groups; Sup. Figure 2.MDA-MB-453 cells with an inducible TRβ vector were treated with three doses of doxycycline for 48 h,and TRβ expression was evaluated using Western blot. GAPDH was used as positive control. Cells were also treated with doxycycline in combination with DOC or DOX for 6 days and then MTT assays were performed to measure cell viability. Results are expressed as fold change ± SD relative to vehicle control (C) cells.* p < 0.05, **p < 0.01; Sup. Figure 3.DAVID analysis was used to identify significant differential expression of the chemokine signaling pathway. Altered genes included chemokines (CCL2, CX3CL1), Gαi (GNAI1), AC (ADCY2), and PKA (PRKACB) and are denoted with red stars; Sup. Figure 4. HCC2185 cells were treated with H89 or Dox alone or in combination for 4 days and protein expression of TRβ, pPKA, PKA, cleaved caspase 3 and caspase 3 expression evaluated by Western blot analysis; GAPDH was used as a loading control; Sup. Figure 5HCC202 EV or SH cells were transfected with TRE reporterand beta galactosidasefor 24 h, and then treated for an additional 24 h with T3, GC-1, or KB141.Cells were then evaluated for TR transcriptional activity. Results are expressed as fold change ± SD relative to vehicle treated cells. * p < 0.05, **p < 0.01, *** p < 0.001, NS = No Significant; Sup. Figure. 6.A.HCC202 cells were treated with GC-1, KB-141, or vehicle for 5 days and TRβ expression measured using Western blot analysis. GAPDH was used as control. B. HCC202 cells were treated with DOXalone and in combination with GC-1 or KB-141 for 9 days and then MTT assays were formed. Results are expressed as fold change ± SD relative to vehicle treated cells (*** p < 0.001) C. HCC202 cells were treated with DOX or GC-1 alone or in combination for 6 days, and MTT growth assays were performed. * p < 0.05. Supplementary material 1 (PPTX 429 kb)
Rights and permissions
About this article
Cite this article
Gu, G., Gelsomino, L., Covington, K.R. et al. Targeting thyroid hormone receptor beta in triple-negative breast cancer. Breast Cancer Res Treat 150, 535–545 (2015). https://doi.org/10.1007/s10549-015-3354-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3354-y