Abstract
The purpose of the study was to compare breast-conserving therapy (BCT) and mastectomy (M) in BRCA1/2 mutation carriers. Women with invasive breast cancer and a pathogenic mutation in BRCA1 or BRCA2 were included in the study (n = 162). Patients treated with BCT (n = 45) were compared with patients treated with M (n = 118). Endpoints were local recurrence as first recurrence (LR), overall survival (OS), breast cancer death, and distant recurrence. Cumulative incidence was calculated in the presence of competing risks. For calculation of hazard ratios and for multivariable analysis, cause-specific Cox proportional hazards regression was used. Compared to M, BCT was associated with an increased risk of LR in univariable analysis (HR 4.0; 95 % CI 1.6–9.8) and in multivariable analysis adjusting for tumor stage, age, and use of adjuvant chemotherapy (HR 2.9; CI 1.1–7.8). Following M, all local recurrences were seen in the first 5 years after breast cancer diagnosis. Following BCT, the rate of LR continued to be high also after the first 5 years. The cumulative incidence of LR in the BCT group was 15, 25, and 32 % after 5, 10, and 15 years, respectively. There were no significant differences between BCT and M for OS, breast cancer death, or distant recurrence. BRCA1/2 mutation carriers treated with BCT have a high risk of LR, many of which are new primary breast cancers. This must be thoroughly discussed with the patient and is an example of how rapid treatment-focused genetic testing could influence choice of treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The knowledge on how BRCA1/2 mutation carriers should be counseled and treated has evolved continuously over the last two decades [1, 2]. Some important issues remain to be solved, though. One of these is if breast-conserving therapy (BCT) followed by postoperative radiotherapy is as good of an alternative to mastectomy (M) for carriers as it is for other breast cancer patients [3, 4]. Once a carrier has had a breast cancer, the risk of contralateral breast cancer (CBC) is indeed very high [5, 6]. It is reasonable to assume that the risk of new primary breast cancers in the ipsilateral breast is also high if breast tissue is still there for tumor development. On the other hand, radiotherapy and other adjuvant treatments change the microenvironment in the breast and reduce the number of cancer precursors, and could thereby, in addition to reducing the risk of true recurrences, possibly also reduce the number of new primary tumors in the treated breast. It is not always possible to differentiate an ipsilateral event being a true recurrence or a new primary breast cancer. In the following, both are denominated “local recurrences.”
Results from cohort studies and case–control studies have been conflicting regarding the risk of local recurrence as first recurrence (LR) in carriers following BCT [7–17]. Importantly, no study to date has shown a survival difference between mutation carriers and noncarriers treated with BCT or between carriers treated with BCT and carriers treated with M. However, BCT can be associated with other disadvantages, such as the requirement of close follow-up for a long time, most likely a recommendation of adjuvant chemotherapy in case of a LR, and an increased cancer-specific distress. Even though generally well tolerated, also M and bilateral mastectomy (BM) can for some patients be associated with disadvantages, like a negative impact on sexuality and body image [18]. The absolute long-term risk of LR and survival endpoints are of pivotal importance for mutation carriers with newly diagnosed breast cancer to know, in order to be able to make informed decisions about type of surgery.
To further evaluate the appropriateness of BCT in carriers, we conducted a cohort study. The aim of the study was to compare LR and survival between carriers treated with BCT and carriers treated with M.
Materials and methods
Study population
In an institutional database, where all persons that have undergone mutation analysis of the BRCA1 and BRCA2 genes at a single institution in Lund, Sweden, are registered, all women with an invasive breast cancer stage I-III diagnosed between 1975 and 2011 and a pathogenic germline mutation in BRCA1 or BRCA2 were selected. Women with variants of uncertain significance in BRCA1 or BRCA2 were not included. Out of 204 identified patients, 183 had consented to longitudinal follow-up (or, in case they were dead, their next of kin had consented), the others were excluded.
Clinical data were abstracted from medical records and pathology reports and supplemented by information from self-reported questionnaires. TNM stage was classified according to American Joint Committee on Cancer 7th edition.
Patients with a diagnosis of ovarian cancer, except for patients with no ovarian cancer recurrences and ≥10 years elapsed after ovarian cancer diagnosis before breast cancer, were excluded (n = 2). Another 8 patients were excluded as we were not able to retrieve medical records. Of the remaining 173 patients, 11 were treated with partial mastectomy without postoperative radiotherapy; they were excluded. For the present study, 162 patients thus constituted the study population. Only 9 of these patients had the mutation analysis initiated after their death (on archived tissue); for the others it was initiated while they were alive. Due to a small number or patients, BRCA1 (n = 114) and BRCA2 (n = 48) mutation carriers were grouped together for analyses. Vital status was controlled in the Swedish Census Register. Current analyses were based on follow-up information through January 31, 2012.
Study endpoints
Study endpoints were local recurrence as first recurrence (LR), overall survival (OS), breast cancer death, and distant recurrence, for the pre-specified subgroups of patients treated with BCT and M, respectively. If the final surgery was M within 1 year after breast cancer diagnosis, the patient was allocated to M, even if the first surgical procedure was a partial mastectomy. For the analysis of LR, patients were censored at the date of last follow-up, whereas distant or regional spread of cancer (breast cancer, ovarian cancer, or another type of cancer) and death where treated as competing risks. In other words, local recurrences occurring after a regional or a distant recurrence were not considered. Further, patients treated with BCT were censored at the time of prophylactic mastectomy. All cases of LR were invasive. For women with bilateral breast cancer, we were not able to distinguish death or distant recurrence due to the first primary breast cancer from death or distant recurrence due to the second primary breast cancer.
Statistical analyses
Differences in patient, tumor, and treatment characteristics between the BCT group and the M group were tested using Fisher’s exact test. Cumulative incidence curves were calculated for LR in the presence of other recurrences or death as competing risks, and for breast cancer death and distant recurrence in the presence of death of other cause than breast cancer as competing risk. Kaplan–Meier curves were used to illustrate OS. To compare event rates between the treatment groups, cause-specific log-rank tests and Cox regression analyses were used.
The following covariates were selected for multivariable analyses: type of surgery, age at diagnosis, TNM stage, and use of (neo)adjuvant chemotherapy. Age at diagnosis was split at the median to account for nonlinear associations and to make interpretations of the results easier. All tests and confidence intervals were two-tailed. All analyses were conducted using the R statistical package (R 3.1.0), using libraries survival and cmprsk. For the discussion part, p values below 0.05 were considered statistically significant.
Results
Study population
Forty-five patients were treated with BCT and 117 patients had M as final surgery within one year from the breast cancer diagnosis. Patient, tumor, and treatment characteristics are listed and compared between these two groups in Table 1. BCT was common in the time period 1990–1999, M was more common before and after that. Tumors treated with M were more often stage III and less often stage I than tumors treated with BCT. Mean age at diagnosis was 43.3 years in both groups. Patients treated with M more often received (neo)adjuvant chemotherapy (59 vs. 42 %) and adjuvant endocrine therapy (37 vs. 13 %) than patients treated with BCT. M was followed by postoperative radiotherapy in 53 % of the cases. A bilateral prophylactic mastectomy was done by 40 % of the patients in the BCT group, and a contralateral prophylactic mastectomy was done by 44 % of the patients in the M group. In both groups, 67 % underwent a bilateral oophorectomy (Table 1). Out of these 108 oophorectomies, 10 were done prior to breast cancer diagnosis, 39 within two years following breast cancer diagnosis, and 59 at a later date. The mean follow-up for OS was 12.9 years for patients alive at the end of follow-up; 14.9 years in the BCT group and 12.1 years in the M group.
Local recurrence
The mean time at risk for LR was 6.0 years in the BCT group and 8.8 years in the M group. The analysis of LR in the BCT group was based on 11 cases of LR diagnosed at a mean time of 7.6 years after breast cancer diagnosis; out of 11 cases of LR, 9 were isolated and 2 were concurrent with a regional recurrence. In the M group, 9 cases of LR were diagnosed at a mean time of 1.9 years after breast cancer diagnosis; out of 9 cases of LR, 6 were isolated, 1 was concurrent with a regional recurrence, and 2 were concurrent with a distant recurrence.
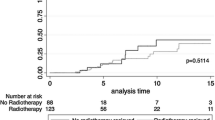
Compared to M, BCT was associated with an increased risk of LR in univariable analysis (HR 4.0; 95 % CI 1.6–9.8) and in multivariable analysis adjusting for tumor stage, age, and use of (neo)adjuvant chemotherapy (HR 2.9; CI 1.1–7.8) (Table 2). In this multivariable model, younger age was associated with a higher risk of LR (<43 vs. ≥43 years: HR 2.7; CI 1.0–7.6), and use of (neo)adjuvant chemotherapy resulted in a point estimate below 1 but a wide confidence interval (HR 0.6; CI 0.2–1.7). Following M, all local recurrences were seen in the first 5 years. As opposed to this, following BCT the rate of LR continued to be high also after the first 5 years. The cumulative risk of LR in the BCT group was 15, 25, and 32 % after 5, 10, and 15 years, respectively. The cumulative risk of LR in the M group was 9 %, after 5, 10 as well as 15 years (Table 3 and Fig. 1).
Overall survival, breast cancer death, and distant recurrence
In univariable analysis, no difference in OS, breast cancer death, or distant recurrence was seen between the BCT group and the M group (Table 3 and Figs. 2, 3 4). In multivariable analysis, adjusting for tumor stage, age at diagnosis, and use of (neo)adjuvant chemotherapy, the hazard ratios were higher, but remained inconclusive (Table 3). The 5-, 10-, and 15-year cumulative incidences are listed in Table 3.
Discussion
In this cohort study, we report that the risk of LR was substantially higher after BCT than after M for BRCA1/2 mutation carriers. The cumulative incidence of LR 15 years after BCT was 32 %, which was significantly higher than after M and is more than two-fold higher than after BCT in the general population, where most tumors are sporadic and the patients on average are older [19, 20]. Apart from type of surgery, younger age was also associated with an increased risk of LR. This can probably be explained by the fact that carriers who get breast cancer when they are very young are likely to have other predisposing or modifying factors of genetic or environmental nature, and thus an increased risk of new primary breast cancers [10, 15, 21].
Most of the case–control and cohort studies that have been conducted to compare the outcome of BCT between carriers and sporadic cases have reported point estimates of LR that have been higher for carriers, although the difference in the majority of the studies have not obtained a significant difference at p values <0.05, and it is therefore difficult to draw firm conclusions from them. The largest such study was reported by Pierce et al. in 2006 [12]. BRCA1/2 mutation carriers were matched with sporadic cases, all treated with BCT. The 15-year risk of LR was 24 % for carriers and 17 % for sporadic cases (p = 0.19). When patients that had done an oophorectomy were excluded from the analysis, carriers had an increased risk of LR (HR 1.9; p = 0.03). The same collaborative group published a different type of study in 2010, which is very similar to ours in study design and is the only previous study that has compared BCT and M for BRCA1/2 mutation carriers [13]. No difference in survival was seen between BCT and M, but patients treated with BCT had a higher risk of LR: the 15-year risk of LR was 23.5 versus 5.5 %. Interestingly, chemotherapy decreased the risk, so in the subgroup of BCT patients treated with chemotherapy, the risk of LR was only 11.9 % after 15 years; a level of risk comparable to what it is for sporadic cases and very relevant for counseling and treatment of carriers today, since a majority of them will receive chemotherapy if diagnosed with a breast cancer. With the limitations of a smaller number of patients and, therefore, no analyses of modifying factors conducted separately in the BCT subgroup, we also found a trend for a decreased risk of LR after use of chemotherapy.
Metcalfe et al. [11] reported data from a large cohort of carriers treated with partial mastectomy without any group for comparison and found the 15-year risk of LR to be 15.8 %. Use of chemotherapy, oophorectomy, and postoperative radiotherapy decreased the risk.
The age at diagnosis and uptake of oophorectomy was similar, but the proportion of patients that received chemotherapy was lower in our cohort than in the above-mentioned studies, which could partly explain the higher absolute risk of LR after BCT seen in our study. A study that reported a higher risk of LR after BCT than what we do, is a retrospective cohort study by Haffty et al., in which the cumulative risk of LR 12 years after BCT was 49 %. Of note, no mutation carriers in that cohort were treated with adjuvant endocrine therapy or oophorectomy, and the mean age at diagnosis was only 34 years [9].
In a recent meta-analysis by Valachis et al., no significant difference in LR after BCT was seen between BRCA1/2 mutation carriers and controls; however, a significant difference was observed when the analysis was restricted to studies with a median follow-up of ≥7 years [17].
A hypothesis that has been corroborated in a number of studies is that early LRs are true recurrences and that late LRs are in fact new primary breast cancers; the latter group probably accounts for the majority of LR [9, 13]. We point out that our cohort consists of mutation carriers ascertained through a cancer genetic center, and a majority of them belong to multiple-case families. Under a model where the risk of late LR is modified by genetic and environmental factors, this risk of LR is higher for these carriers than for carriers ascertained through population-based programs. The decreased risk of LR conferred by adjuvant therapy could be mediated not only through killing of breast cancer cells from the primary tumor, but also through an effect on breast cancer precursors and changes in the microenvironment of the breast, making new tumor formation less likely. The difference in the risk of LR between carriers and sporadic cases is less clear in studies where more adjuvant treatment has been used and a majority of the patients have done an oophorectomy, supporting the notion that these measures reduce the risk for new primary breast cancers in the ipsilateral, as well as the contralateral, breast.
In our study, the survival comparisons between BCT and M should be interpreted with caution. M was more common than BCT in the time period 1975–1989. Patients who died before they were known mutation carriers could be included in the study, but patients that were still alive when BRCA1/2 testing was introduced in the mid-90s were probably more likely to be included, resulting in survivorship bias. M was also more common in the time period 2000–2011, when adjuvant treatment was more common and modern than before.
Neither our cohort nor any of the other cohorts in published studies is large enough to properly adjust for all possible biases and confounders. Furthermore, in observational studies of surgical decisions, bias can never be fully accounted for.
In the general population, large randomized trials of breast cancer patients have not shown a difference in survival between BCT and M. Still, in the general population, patients that are diagnosed with a LR have an inferior survival compared to patients without a LR [22]. Despite the fact that some studies of BRCA1/2 mutation carriers have shown a very high risk of LR, no study to date has shown a difference in survival. This contradiction could possibly be explained by a larger proportion of new primary breast cancers among carriers, which are less aggressive than early true recurrences, and more often curable [13]. However, given large enough sample sizes and a cohort with a high rate of LR following BCT, a difference in long-term survival between BCT and M would be expected for carriers, since not all new primary breast cancers are curable.
Apart from the inability to make reliable survival comparisons, there are other limitations to our study. First, the follow-up is too short to estimate cumulative lifetime risks of LR, which has not been done in previous studies either. Second, a small number of patients result in point estimates with wide confidence intervals and the inability to include some potentially important variables in the multivariable models. Still, our study confirms some of the findings from the larger collaborative study by Pierce et al. Third, by including breast cancer patients from 1975 and onwards, the proportion of patients receiving adjuvant treatment is lower than what it is today, which can overestimate the risk of LR after BCT. Fourth, we were not able to separate true local recurrences from new primary breast cancers by means of pathology or localization in the breast.
The number of mutation carriers opting for M and BM has increased over the last decade, and the number opting for BCT has decreased [23]; the trend is expected to continue further with evidence of a survival benefit with BM now starting to emerge [5, 24, 25]. In the future, studies on the risk of LR after BCT in mutation carriers will therefore be harder to carry out and randomized trials are very unlikely. Still, there is a need for more studies, since BCT is considered an acceptable option for mutation carriers today. Data from retrospective studies can give relatively unbiased estimates of LR, but cannot properly measure psychosocial endpoints. In future studies, for an optimal generalizability to current standards, a very high proportion of patients treated with chemotherapy and prophylactic oophorectomy is needful. Furthermore, a long-term follow-up is demanded for estimation of cumulative lifetime risks of LR, which are pivotal for carriers with newly diagnosed breast cancer to know in order to make informed decisions about type of surgery.
In conclusion, BRCA1/2 mutation carriers treated with BCT and who resemble our cohort regarding ascertainment, age at diagnosis, adjuvant treatment, uptake of oophorectomy etc., have a high risk of LR, many of which are probably new primary breast cancers. This must be thoroughly discussed with the patient and is an example of how rapid treatment-focused genetic testing could influence choice of treatment.
References
Tung N (2011) Management of women with BRCA mutations: a 41-year-old woman with a BRCA mutation and a recent history of breast cancer. JAMA, J Am Med Assoc 305(21):2211–2220. doi:10.1001/jama.2011.678
Lynch HT, Snyder C, Casey MJ (2013) Hereditary ovarian and breast cancer: what have we learned? Annals of oncology 24(Suppl 8):viii83–viii95. doi:10.1093/annonc/mdt313
Bordeleau L, Panchal S, Goodwin P (2010) Prognosis of BRCA-associated breast cancer: a summary of evidence. Breast Cancer Res Treat 119(1):13–24. doi:10.1007/s10549-009-0566-z
Croshaw RL, Marshall ML, Williams TL, Erb KM, Julian TB (2011) Prophylactic and Therapeutic Breast Conservation in BRCA1/2 mutation carriers. Int J Breast cancer 2011:481563. doi:10.4061/2011/481563
Evans DG, Ingham SL, Baildam A, Ross GL, Lalloo F, Buchan I, Howell A (2013) Contralateral mastectomy improves survival in women with BRCA1/2-associated breast cancer. Breast Cancer Res Treat 140(1):135–142. doi:10.1007/s10549-013-2583-1
Rhiem K, Engel C, Graeser M, Zachariae S, Kast K, Kiechle M, Ditsch N, Janni W, Mundhenke C, Golatta M, Varga D, Preisler-Adams S, Heinrich T, Bick U, Gadzicki D, Briest S, Meindl A, Schmutzler RK (2012) The risk of contralateral breast cancer in patients from BRCA1/2 negative high risk families as compared to patients from BRCA1 or BRCA2 positive families: a retrospective cohort study. BCR: Breast Cancer Res 14(6):R156. doi:10.1186/bcr3369
Brekelmans CT, Tilanus-Linthorst MM, Seynaeve C, vd Ouweland A, Menke-Pluymers MB, Bartels CC, Kriege M, van Geel AN, Burger CW, Eggermont AM, Meijers-Heijboer H, Klijn JG (2007) Tumour characteristics, survival and prognostic factors of hereditary breast cancer from BRCA2-, BRCA1- and non-BRCA1/2 families as compared to sporadic breast cancer cases. Eur J Cancer 43(5):867–876. doi:10.1016/j.ejca.2006.12.009 Oxford, England: 1990
Garcia-Etienne CA, Barile M, Gentilini OD, Botteri E, Rotmensz N, Sagona A, Farante G, Galimberti V, Luini A, Veronesi P, Bonanni B (2009) Breast-conserving surgery in BRCA1/2 mutation carriers: are we approaching an answer? Ann Surg Oncol 16(12):3380–3387. doi:10.1245/s10434-009-0638-7
Haffty BG, Harrold E, Khan AJ, Pathare P, Smith TE, Turner BC, Glazer PM, Ward B, Carter D, Matloff E, Bale AE, Alvarez-Franco M (2002) Outcome of conservatively managed early-onset breast cancer by BRCA1/2 status. Lancet 359(9316):1471–1477. doi:10.1016/s0140-6736(02)08434-9
Kirova YM, Savignoni A, Sigal-Zafrani B, de La Rochefordiere A, Salmon RJ, This P, Asselain B, Stoppa-Lyonnet D, Fourquet A (2010) Is the breast-conserving treatment with radiotherapy appropriate in BRCA1/2 mutation carriers? Long-term results and review of the literature. Breast Cancer Res Treat 120(1):119–126. doi:10.1007/s10549-009-0685-6
Metcalfe K, Lynch HT, Ghadirian P, Tung N, Kim-Sing C, Olopade OI, Domchek S, Eisen A, Foulkes WD, Rosen B, Vesprini D, Sun P, Narod SA (2011) Risk of ipsilateral breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat 127(1):287–296. doi:10.1007/s10549-010-1336-7
Pierce LJ, Levin AM, Rebbeck TR, Ben-David MA, Friedman E, Solin LJ, Harris EE, Gaffney DK, Haffty BG, Dawson LA, Narod SA, Olivotto IA, Eisen A, Whelan TJ, Olopade OI, Isaacs C, Merajver SD, Wong JS, Garber JE, Weber BL (2006) Ten-year multi-institutional results of breast-conserving surgery and radiotherapy in BRCA1/2-associated stage I/II breast cancer. J Clin Oncol 24(16):2437–2443. doi:10.1200/jco.2005.02.7888
Pierce LJ, Phillips KA, Griffith KA, Buys S, Gaffney DK, Moran MS, Haffty BG, Ben-David M, Kaufman B, Garber JE, Merajver SD, Balmana J, Meirovitz A, Domchek SM (2010) Local therapy in BRCA1 and BRCA2 mutation carriers with operable breast cancer: comparison of breast conservation and mastectomy. Breast Cancer Res Treat 121(2):389–398. doi:10.1007/s10549-010-0894-z
Robson ME, Chappuis PO, Satagopan J, Wong N, Boyd J, Goffin JR, Hudis C, Roberge D, Norton L, Begin LR, Offit K, Foulkes WD (2004) A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. BCR: Breast Cancer Res 6(1):R8–R17. doi:10.1186/bcr658
Robson M, Levin D, Federici M, Satagopan J, Bogolminy F, Heerdt A, Borgen P, McCormick B, Hudis C, Norton L, Boyd J, Offit K (1999) Breast conservation therapy for invasive breast cancer in Ashkenazi women with BRCA gene founder mutations. J Natl Cancer Inst 91(24):2112–2117
Seynaeve C, Verhoog LC, van de Bosch LM, van Geel AN, Menke-Pluymers M, Meijers-Heijboer EJ, van den Ouweland AM, Wagner A, Creutzberg CL, Niermeijer MF, Klijn JG, Brekelmans CT (2004) Ipsilateral breast tumour recurrence in hereditary breast cancer following breast-conserving therapy. Eur J Cancer 40(8):1150–1158. doi:10.1016/j.ejca.2004.01.017 Oxford, England : 1990
Valachis A, Nearchou AD, Lind P (2014) Surgical management of breast cancer in BRCA-mutation carriers: a systematic review and meta-analysis. Breast Cancer Res Treat. doi:10.1007/s10549-014-2890-1
Brandberg Y, Sandelin K, Erikson S, Jurell G, Liljegren A, Lindblom A, Linden A, von Wachenfeldt A, Wickman M, Arver B (2008) Psychological reactions, quality of life, and body image after bilateral prophylactic mastectomy in women at high risk for breast cancer: a prospective 1-year follow-up study. J Clin Oncol 26(24):3943–3949. doi:10.1200/jco.2007.13.9568
Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N (2002) Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. New Engl J Med 347(16):1233–1241. doi:10.1056/NEJMoa022152
Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, Aguilar M, Marubini E (2002) Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. New Engl J Med 347(16):1227–1232. doi:10.1056/NEJMoa020989
Mavaddat N, Peock S, Frost D, Ellis S, Platte R, Fineberg E, Evans DG, Izatt L, Eeles RA, Adlard J, Davidson R, Eccles D, Cole T, Cook J, Brewer C, Tischkowitz M, Douglas F, Hodgson S, Walker L, Porteous ME, Morrison PJ, Side LE, Kennedy MJ, Houghton C, Donaldson A, Rogers MT, Dorkins H, Miedzybrodzka Z, Gregory H, Eason J, Barwell J, McCann E, Murray A, Antoniou AC, Easton DF (2013) Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst 105(11):812–822. doi:10.1093/jnci/djt095
Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y (2005) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366(9503):2087–2106. doi:10.1016/s0140-6736(05)67887-7
McGuire KP, Santillan AA, Kaur P, Meade T, Parbhoo J, Mathias M, Shamehdi C, Davis M, Ramos D, Cox CE (2009) Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol 16(10):2682–2690. doi:10.1245/s10434-009-0635-x
Heemskerk-Gerritsen BA, Menke-Pluijmers MB, Jager A, Tilanus-Linthorst MM, Koppert LB, Obdeijn IM, van Deurzen CH, Collee JM, Seynaeve C, Hooning MJ (2013) Substantial breast cancer risk reduction and potential survival benefit after bilateral mastectomy when compared with surveillance in healthy BRCA1 and BRCA2 mutation carriers: a prospective analysis. Ann Oncol 24(8):2029–2035. doi:10.1093/annonc/mdt134
Metcalfe K, Gershman S, Ghadirian P, Lynch HT, Snyder C, Tung N, Kim-Sing C, Eisen A, Foulkes WD, Rosen B, Sun P, Narod SA (2014) Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis. BMJ (Clin Res Ed) 348:g226. doi:10.1136/bmj.g226
Acknowledgments
The study was approved by the Regional Ethical Review Board in Lund, and complies with the current laws of Sweden. The research was funded by grants from Skåne County Counsil’s Research and Development Foundation, The Swedish Breast Cancer Association (BRO), The Swedish Cancer Society, and BioCARE.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Nilsson, M.P., Hartman, L., Kristoffersson, U. et al. High risk of in-breast tumor recurrence after BRCA1/2-associated breast cancer. Breast Cancer Res Treat 147, 571–578 (2014). https://doi.org/10.1007/s10549-014-3115-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3115-3