Abstract
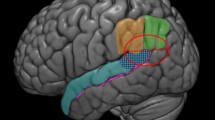
Event related potentials (ERPs) provide precise temporal information about cognitive processing, but with poor spatial resolution, while functional magnetic resonance imaging (fMRI) reliably identifies brain areas involved, but with poor temporal resolution. Here we use fMRI to guide source localization of the ERPs at different times for studying the temporal dynamics of the neural system for recognizing familiar faces. fMRI activation areas were defined in a previous experiment applying the same paradigm used for ERPs. The Bayesian model averaging (BMA) method was used to estimate the generators of the ERPs to unfamiliar, visually familiar, and personally-familiar faces constraining the model by fMRI activation results. For this, higher prior probabilities in the solution space were assigned to the fMRI-defined regions, which included face-selective areas and other areas related to “person knowledge” retrieval. Source analysis was carried out in three-time windows: early (150–210 ms), middle (300–380 ms) and late (460–580 ms). The early and middle responses were generated in fMRI-defined areas for all face categories, while these areas do not contribute to the late response. Different areas contributed to the generation of the early and middle ERPs elicited by unfamiliar faces: fusiform (Fus), inferior occipital, superior temporal sulcus and the posterior cingulate (PC) cortices. For familiar faces, the contributing areas were Fus, PC and anterior temporal areas for visually familiar faces, with the addition of the medial orbitofrontal areas and other frontal structures for personally-significant faces. For both unfamiliar and familiar faces, more extended and reliable involvement of contributing areas were obtained for the middle compare with early time window. Our fMRI guide ERP source analysis suggested the recruitment of person-knowledge processing areas as early as 150–210 ms after stimulus onset during recognition of personally-familiar faces. We concluded that fMRI-constrained BMA source analysis provide information regarding the temporal-dynamics in the neural system for cognitive processsing.






Similar content being viewed by others
References
Allison T, Ginter H, McCarthy G, Nobre AC, Puce A, Luby M, Spencer DD (1994) Face recognition in human extrastriate cortex. J Neurophysiol 71:821–825
Allison T, Puce A, Spencer DD, McCarthy G (1999) Electrophysiological studies of human face perception. I: potentials generated in occipitotemporal cortex by face and non-face stimuli. Cereb Cortex 9:415–430
Barbeau EJ, Taylor MJ, Regis J, Marquis P, Chauvel P, Liegeois-Chauvel C (2008) Spatio temporal dynamics of face recognition. Cereb Cortex 18:997–1009
Bentin S, Deouell LY (2000) Structural encoding and identification in face processing: erp evidence for separate mechanisms. Cogn Neuropsychol 17:35–55
Bobes MA, Lopera F, Comas LD, Galan L, Carbonell F, Bringas ML, Valdes-Sosa M (2004) Brain potentials reflect residual face processing in a case of prosopagnosia. Cogn Neuropsychol 21:691–718
Bobes MA, Quinonez I, Perez J, Leon I, Valdes-Sosa M (2007) Brain potentials reflect access to visual and emotional memories for faces. Biol Psychol 75:146–153
Bobes MA, Lage CA, Quinones I, Garcia L, Valdes-Sosa M (2013) Timing and tuning for familiarity of cortical responses to faces. PLoS ONE 8:e76100
Brambati SM, Benoit S, Monetta L, Belleville S, Joubert S (2010) The role of the left anterior temporal lobe in the semantic processing of famous faces. Neuroimage 53:674–681
Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ (1999) Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging 18:32–42
Caldara R, Thut G, Servoir P, Michel CM, Bovet P, Renault B (2003) Face versus non-face object perception and the ‘other-race’ effect: a spatio-temporal event-related potential study. Clin Neurophysiol 114:515–528
Corrigan NM, Richards T, Webb SJ, Murias M, Merkle K, Kleinhans NM, Johnson LC, Poliakov A, Aylward E, Dawson G (2009) An investigation of the relationship between fMRI and ERP source localized measurements of brain activity during face processing. Brain Topogr 22:83–96
Dale AM, Sereno MI (1993) Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci 5:162–176
Dalrymple KA, Oruc I, Duchaine B, Pancaroglu R, Fox CJ, Iaria G, Handy TC, Barton JJ (2011) The anatomic basis of the right face-selective N170 IN acquired prosopagnosia: a combined ERP/fMRI study. Neuropsychologia 49:2553–2563
Deffke I, Sander T, Heidenreich J, Sommer W, Curio G, Trahms L, Lueschow A (2007) MEG/EEG sources of the 170-ms response to faces are co-localized in the fusiform gyrus. Neuroimage 35:1495–1501
Dietl T, Trautner P, Staedtgen M, Vannuchi M, Mecklinger A, Grunwald T, Clusmann H, Elger CE, Kurthen M (2005) Processing of famous faces and medial temporal lobe event-related potentials: a depth electrode study. Neuroimage 25:401–407
Dobel C, Putsche C, Zwitserlood P, Junghofer M (2008) Early left-hemispheric dysfunction of face processing in congenital prosopagnosia: an MEG study. PLoS ONE 3:e2326
Eimer M (2000a) Event-related brain potentials distinguish processing stages involved in face perception and recognition. Clin Neurophysiol 111:694–705
Eimer M (2000b) Effects of face inversion on the structural encoding and recognition of faces. Evidence from event-related brain potentials. Brain Res Cogn Brain Res 10:145–158
Eimer M (2011) The face-sensitivity of the n170 component. Front Hum Neurosci 5:119
Eimer M, Kiss M, Nicholas S (2010) Response profile of the face-sensitive N170 component: a rapid adaptation study. Cereb Cortex 20:2442–2452
Elfgren C, van WD, Passant, Larsson U, Mannfolk EM, Fransson P P (2006) fMRI activity in the medial temporal lobe during famous face processing. Neuroimage 30:609–616
Eryilmaz HH, Duru AD, Parlak B, Ademoglu A, Demiralp T (2007) Neuroimaging of event related brain potentials (ERP) using fMRI and dipole source reconstruction. Conf Proc IEEE Eng Med Biol Soc 2007:3384–3387
Gao Z, Goldstein A, Harpaz Y, Hansel M, Zion-Golumbic E, Bentin S (2013) A magnetoencephalographic study of face processing: M170, gamma-band oscillations and source localization. Hum Brain Mapp 34:1783–1795
Genetti M, Khateb A, Heinzer S, Michel CM, Pegna AJ (2009) Temporal dynamics of awareness for facial identity revealed with ERP. Brain Cogn 69:296–305
Gobbini MI, Haxby JV (2007) Neural systems for recognition of familiar faces. Neuropsychologia 45:32–41
Gobbini MI, Leibenluft E, Santiago N, Haxby JV (2004) Social and emotional attachment in the neural representation of faces. Neuroimage 22:1628–1635
Gorno-Tempini ML, Price CJ (2001) Identification of famous faces and buildings: a functional neuroimaging study of semantically unique items. Brain 124:2087–2097
Halgren E, Baudena P, Heit G, Clarke JM, Marinkovic K, Clarke M (1994) Spatio-temporal stages in face and word processing. I. Depth-recorded potentials in the human occipital, temporal and parietal lobes [corrected]. J Physiol Paris 88:1–50
Haxby JV, Hoffman EA, Gobbini MI (2000) The distributed human neural system for face perception. Trends Cogn Sci 4:223–233
Henson RN, Goshen-Gottstein Y, Ganel T, Otten LJ, Quayle A, Rugg MD (2003) Electrophysiological and haemodynamic correlates of face perception, recognition and priming. Cereb Cortex 13:793–805
Henson RN, Mattout J, Singh KD, Barnes GR, Hillebrand A, Friston K (2007) Population-level inferences for distributed MEG source localization under multiple constraints: application to face-evoked fields. Neuroimage 38:422–438
Henson RN, Mouchlianitis E, Friston KJ (2009) MEG and EEG data fusion: simultaneous localisation of face-evoked responses. Neuroimage 47:581–589
Herrmann MJ, Ehlis AC, Muehlberger A, Fallgatter AJ (2005) Source localization of early stages of face processing. Brain Topogr 18:77–85
Horovitz SG, Rossion B, Skudlarski P, Gore JC (2004) Parametric design and correlational analyses help integrating fMRI and electrophysiological data during face processing. Neuroimage 22:1587–1595
Iidaka T, Matsumoto A, Haneda K, Okada T, Sadato N (2006) Hemodynamic and electrophysiological relationship involved in human face processing: evidence from a combined fMRI-ERP study. Brain Cogn 60:176–186
Itier RJ, Taylor MJ (2004) Source analysis of the N170 to faces and objects. Neuroreport 15:1261–1265
Jemel B, George N, Olivares E, Fiori N, Renault B (1999) Event-related potentials to structural familiar face incongruity processing. Psychophysiology 36:437–452
Jemel B, Coutya J, Langer C, Roy S (2009) From upright to upside-down presentation: a spatio-temporal ERP study of the parametric effect of rotation on face and house processing. BMC Neurosci 10:100
Joyce C, Rossion B (2005) The face-sensitive N170 and VPP components manifest the same brain processes: the effect of reference electrode site. Clin Neurophysiol 116:2613–2631
Kaufmann JM, Schweinberger SR, Burton AM (2009) N250 ERP correlates of the acquisition of face representations across different images. J Cogn Neurosci 21:625–641
Latinus M, Taylor MJ (2006) Face processing stages: impact of difficulty and the separation of effects. Brain Res 1123:179–187
Leveroni CL, Seidenberg M, Mayer AR, Mead LA, Binder JR, Rao SM (2000) Neural systems underlying the recognition of familiar and newly learned faces. J Neurosci 20:878–886
Linkenkaer-Hansen K, Palva JM, Sams M, Hietanen JK, Aronen HJ, Ilmoniemi RJ (1998) Face-selective processing in human extrastriate cortex around 120 ms after stimulus onset revealed by magneto- and electroencephalography. Neurosci Lett 253:147–150
MacKay DJ (1992) Bayesian interpolation. Neural Comput 4(3):415–447
Melie-García L, Trujillo-Barreto NJ, Martínez-Montes E, Koenig T, Valdés-Sosa PA (2004) EEG imaging via BMA with fMRI pre-defined prior model probabilities. In: Human Brain Mapping, Budapest, June
Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave de PR (2004) EEG source imaging. Clin Neurophysiol 115:2195–2222
Mnatsakanian EV, Tarkka IM (2004) Familiar-face recognition and comparison: source analysis of scalp-recorded event-related potentials. Clin Neurophysiol 115:880–886
Oostendorp TF, Delbeke J, Stegeman DF (2000) The conductivity of the human skull: results of in vivo and in vitro measurements. IEEE Trans Biomed Eng 47:1487–1492
Oruc I, Cheung T, Dalrymple K, Fox C, Iaria G, Handy T, Barton J (2010) Residual face-selectivity of the N170 and M170 is related to the status of the occipital and fusiform face areas in acquired prosopagnosia. J Vision 10(7), article 585. https://doi.org/10.1167/10.7.585
Penny WD, Mattout J, Trujillo-Barreto NJ (2006) Chap. 35. Bayesian model selection & averaging. In: Friston KJ et al (eds) Statistical parametric mapping: the analysis of functional brain images. Academic Press, Oxford, pp 454–467
Pizzagalli DA, Lehmann D, Hendrick AM, Regard M, Pascual-Marqui RD, Davidson RJ (2002) Affective judgments of faces modulate early activity (approximately 160 ms) within the fusiform gyri. Neuroimage 16:663–677
Pourtois G, Dan ES, Grandjean D, Sander D, Vuilleumier P (2005a) Enhanced extrastriate visual response to bandpass spatial frequency filtered fearful faces: time course and topographic evoked-potentials mapping. Hum Brain Mapp 26:65–79
Pourtois G, Schwartz S, Seghier ML, Lazeyras F, Vuilleumier P (2005b) View-independent coding of face identity in frontal and temporal cortices is modulated by familiarity: an event-related fMRI study. Neuroimage 24:1214–1224
Puce A, Allison T, Asgari M, Gore JC, McCarthy G (1996) Differential sensitivity of human visual cortex to faces, letterstrings, and textures: a functional magnetic resonance imaging study. J Neurosci 16:5205–5215
Puce A, Allison T, McCarthy G (1999) Electrophysiological studies of human face perception. III: effects of top-down processing on face-specific potentials. Cereb Cortex 9:445–458
Rosenblatt JD, Vink M, Benjamini Y (2014) Revisiting multi-subject random effects in fMRI: advocating prevalence estimation. Neuroimage 84:113–121
Rossion B, Campanella S, Gomez CM, Delinte A, Debatisse D, Liard L, Dubois S, Bruyer R, Crommelinck M, Guerit JM (1999) Task modulation of brain activity related to familiar and unfamiliar face processing: an ERP study. Clin Neurophysiol 110:449–462
Rossion B, Joyce CA, Cottrell GW, Tarr MJ (2003) Early lateralization and orientation tuning for face, word, and object processing in the visual cortex. Neuroimage 20:1609–1624
Rotshtein P, Henson RN, Treves A, Driver J, Dolan RJ (2005) Morphing Marilyn into Maggie dissociates physical and identity face representations in the brain. Nat Neurosci 8:107–113
Schweinberger SR (2011) Neurophysiological correlates of face recognition. In: Calder AJ, Rhodes G, Johnson MH, Haxby JV (eds) The handbook of face perception. Oxford University Press, Oxford
Schweinberger SR, Pickering EC, Jentzsch I, Burton AM, Kaufmann JM (2002) Event-related brain potential evidence for a response of inferior temporal cortex to familiar face repetitions. Brain Res Cogn Brain Res 14:398–409
Shibata T, Nishijo H, Tamura R, Miyamoto K, Eifuku S, Endo S, Ono T (2002) Generators of visual evoked potentials for faces and eyes in the human brain as determined by dipole localization. Brain Topogr 15:51–63
Tarkka IM, Mnatsakanian EV (2003) Functional specialization in the human frontal cortex observed during task anticipation. Neuropsychobiology 48:102–110
Trautmann-Lengsfeld SA, Domínguez-Borràs J, Escera C, Herrmann M, Fehr T (2013). The perception of dynamic and static facial expressions of happiness and disgust investigated by ERPs and fMRI constrained source analysis. PLoS ONE, 8(6):e66997
Trujillo-Barreto NJ, Aubert-Vazquez E, Valdes-Sosa PA (2004) Bayesian model averaging in EEG/MEG imaging. Neuroimage 21:1300–1319
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289
Xu L, Johnson TD, Nichols TE, Nee DE (2009) Modeling inter-subject variability in FMRI activation location: a Bayesian hierarchical spatial model. Biometrics 65:1041–1051
Zhang Y, van Drongelen W, He B (2006) Estimation of in vivo brain-to-skull conductivity ratio in humans. Appl Phys Lett 89:223903
Acknowledgements
The present study was partly supported by a scientific collaboration program between the Cuban Neuroscience Center and the Autonomous University of Madrid and by the Grant No. 81330032 from the National Nature Science Foundation of China NSFC.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Handling Editor: Laura Astolfi.
This is one of several papers published together in Brain Topography on the “Special Issue: Controversies in EEG Source Analysis”.
Rights and permissions
About this article
Cite this article
Bobes, M.A., Lage-Castellanos, A., Olivares, E.I. et al. ERP Source Analysis Guided by fMRI During Familiar Face Processing. Brain Topogr 32, 720–740 (2019). https://doi.org/10.1007/s10548-018-0619-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-018-0619-x