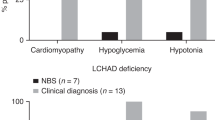
Abstract
Background
Patients with very long chain acyl-CoA dehydrogenase deficiency (VLCADD), a long chain fatty acid oxidation disorder, are traditionally treated with a long chain triglyceride (LCT) restricted and medium chain triglyceride (MCT) supplemented diet. Introduction of VLCADD in newborn screening (NBS) programs has led to the identification of asymptomatic newborns with VLCADD, who may have a more attenuated phenotype and may not need dietary adjustments.
Objective
To define dietary strategies for individuals with VLCADD based on the predicted phenotype.
Method
We evaluated long-term dietary histories of a cohort of individuals diagnosed with VLCADD identified before the introduction of VLCADD in NBS and their beta-oxidation (LC-FAO) flux score (rate of oleate oxidation) in cultured skin fibroblasts in relation to the clinical outcome. Based on these results a dietary strategy is proposed.
Results
Sixteen individuals with VLCADD were included. One had an LC-FAO flux score >90%, was not on a restricted diet and is asymptomatic to date. Four patients had an LC-FAO flux score <10%, and significant VLCADD related symptoms despite the use of strict diets including LCT restriction, MCT supplementation and nocturnal gastric drip feeding. Patients with an LC-FAO flux score between 10 and 90% (n = 11) showed a more heterogeneous phenotype.
Conclusions
This study shows that a strict diet cannot prevent poor clinical outcome in severely affected patients and that the LC-FAO flux is a good predictor of clinical outcome in individuals with VLCADD identified before its introduction in NBS. Hereby, we propose an individualized dietary strategy based on the LC-FAO flux score.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Very long chain acyl-CoA dehydrogenase deficiency (VLCADD) is an autosomal recessive inherited disorder of mitochondrial long-chain fatty acid beta-oxidation (OMIM 201475) in which energy homeostasis is compromised and toxic intermediates accumulate. Patients may present with hypoglycemia, rhabdomyolysis, hepatomegaly, and (cardio) myopathy (Andresen et al 1999; Bonnet et al 1999; Saudubray et al 1999; Wanders et al 1999; Spiekerkoetter et al 2010; Baruteau et al 2013). Traditionally, treatment of VLCADD is aimed at preventing catabolism by avoidance of fasting (Arnold et al 2009; Spiekerkoetter et al 2009). In addition, a long chain triglycerides (LCT) restricted diet, supplemented with medium chain triglycerides (MCT), is generally advised in order to bypass long chain fatty acid oxidation for energy production (Bach and Babayan 1982; Solis and Singh 2002; Arnold et al 2009; Spiekerkoetter et al 2009; Behrend et al 2012).
In the past few decades, VLCADD has been incorporated into newborn screening (NBS) programs in many countries worldwide. This has resulted in the detection of individuals with a diagnosis of VLCADD confirmed by molecular and/or enzymatic studies that are asymptomatic or have less severe phenotypes. It has been suggested that patients with less severe phenotypes might be treated by avoidance of long-term fasting only, without LCT restriction and MCT supplementation (Spiekerkoetter et al 2010; Hoffmann et al 2012). However, the published consensus guidelines advise that all patients over 12 months of age should be treated with MCT supplementation (Arnold et al 2009; Spiekerkoetter et al 2009). Since NBS will not only detect symptomatic but also asymptomatic individuals, there is an urgent need for early prediction of the phenotypic severity of the disorder thus allowing personalized treatment. Unfortunately, genotype-phenotype correlation is poor in VLCADD as missense variants of unknown or uncertain significance are frequent in both the severe and milder phenotypes (Hoffmann et al 2012).
Previously, we reported a strong correlation between the long chain fatty acid oxidation (LC-FAO) flux score (i.e., the rate of oleate beta-oxidation in cultured skin fibroblasts) of VLCADD patients and their clinical outcome (Diekman et al 2015). In order to define a dietary strategy for individuals with different phenotypes of VLCADD, we retrospectively analyzed the dietary treatment strategies and clinical outcomes of patients with VLCADD identified before the introduction of VLCADD in the Dutch NBS panel and related these data with the results of LC-FAO flux measurement. Based on this evaluation we propose a novel dietary strategy for patients identified with VLCADD by NBS.
Subjects and methods
In the Netherlands, all patients with inherited metabolic diseases are registered in the Dutch Diagnosis Registration Metabolic Diseases (DDRMD). The DDRMD contains 27 VLCADD patients born before the inclusion of VLCADD in the Dutch NBS panel in 2007. Of these patients, four are deceased and for none of them fibroblasts were available for LC-FAO flux examination. Seven patients were lost for follow-up. For this study the remaining 16 patients (nine males, seven females) were included with a median age at inclusion 19.5 years (range 13–45 years).
In the Netherlands, all patients with inherited metabolic diseases are treated and followed up in metabolic centers, located in six academic hospitals. In addition, patients with FAO disorders are regularly examined in the Dutch FAO expertise center at the University Medical Center Utrecht by a multidisciplinary team consisting of a metabolic specialist, a research dietician, a neurologist, a physical therapists and a cardiologist.
All patients have a confirmed diagnosis based on deficient VLCAD enzymatic activity in lymphocytes and/or cultured fibroblasts and the presence of biallelic mutations in the ACADVL gene (OMIM 609575).
LC-FAO flux score
The LC-FAO flux score in cultured skin fibroblasts was measured as described previously (Manning et al 1990; Olpin et al 1997, 1999; Diekman et al 2015). The LC-FAO flux score is expressed as a percentage of the mean activity (nanomoles of fatty acid oxidized per hour per milligram of cellular protein) in healthy control skin fibroblasts measured in the same experiment. VLCAD enzyme activity in lymphocytes is measured as described previously (Wanders et al 2010). Details of time and cause of diagnosis, enzyme activity, mutations, and LC-FAO flux scores are presented in Tables 1 and 2.
Nutritional data
Patients completed a 3-day food journal before visiting the clinic, including two weekdays and one weekend day. Information was collected on the maximal feeding pause, use of medical nutrition and supplements and dietary adjustments during illness or sport. Dietary intake was analyzed using the Dutch Food Composition Dataset (RIVM 2011). Adjustments in calories for MCT fat, as this contains 8.3 kcal/g instead of 9 kcal/g for LCT fat, were calculated manually.
Clinical severity score (CSS)
We used the severity score as previously described by Diekman et al (Diekman et al 2015). In brief, this score is based on key parameters in three organ domains: history of hypoglycemia (reported glucose <2.5 mmol/L), cardiac involvement (cardiomyopathy as documented by abnormal results on echocardiography with left or right ventricular wall thickness of at least one segment >2 SD, corrected for age or arrhythmia (as documented ECG) and myopathy (as documented CK >250 U/L (ref values 70–170 U/L)) and a history including at least two of the following symptoms: myoglobinuria, myalgia, exercise intolerance compared to age matched reference values, muscle weakness (medical research council (MRC) grade 4 or less), and/or frequent fatigue. A score of one point was given for each criterion (hypoglycemia, cardiac involvement, and myopathy) present, resulting in a CSS between 0 and 3.
Ethics
The study was approved by the medical ethics committee of the University Medical Centre Utrecht (METC 10–430). All patients gave written informed consent for participation in this study.
Statistics
Statistical analyses were performed using IBM SPSS Statistics version 21 (IBM corp., Armonk, NY, USA). The median is reported for continuous parameters, such as height and BMI. Reported percentages are the valid percentages, missing subjects were excluded from the analyses.
Results
LC-FAO flux score, dietary treatment, and clinical severity score
Details on the LC-FAO flux score and clinical outcome are shown in Tables 1, 3, and Suppl. Table 1.
An LC-FAO flux score of <10% (median: 6.0% (range 5.6–6.6)) was detected in fibroblasts of four patients. All four had been on nocturnal gastric drip feeding during their early years of life and were still on a strict diet containing <20 energy percent (E%) LCT supplemented with MCT, with a limited maximal feeding pause (Tables 2 and 3). All four had a CSS of 3. In addition, they still had recurrent symptoms and were frequently hospitalized for metabolic crises. Finally, all had or had had adjusted school schedules or were unemployed because of health issues.
In contrast, the one patient with an LC-FAO flux score of >90% had no symptoms (CSS of 0), had not used any dietary treatment, and followed general education.
The 11 patients with LC-FAO flux scores between 10 and 90% showed a more heterogeneous phenotype. In this group, we did not find a correlation between LC-FAO flux score and CSS. LCT restriction and MCT supplementation had been started in seven patients, and three were still on this diet at the time of this study. In this cohort, all patients with a calculated daily intake of LCT ≤20 E% (3–20 E%, n = 7) are supplemented with MCT (1–32 E%) (Table 2). Four patients returned to a normal diet after initial LCT restriction and MCT supplementation on their own (n = 2) or doctor’s initiative after an asymptomatic period (Table 3).
We were able to phase out MCT in two asymptomatic patients without adverse events. Reducing MCT was done stepwise with either home controlled monitoring or hospital controlled monitoring of CK and acylcarnitines. As an example, patient 14, was supplemented with MCT until the age of 4, but MCT dose was gradually reduced in steps of 2 weeks with 5% decrease of dosage and weekly monitoring of CK and acylcarnitine profiles (mother took blood at home). No clinical deterioration was observed and the family reported a serious improvement in quality of life when dietary restrictions were alleviated. It is not possible to correlate this to clinical outcome, because the patients were already in a good clinical condition when MCT was reduced.
In contrast, the severe patients who are currently still on an LCT-restricted/MCT-supplemented diet experience symptoms on a regular basis. We have never deliberately tried to reduce the amount of MCT in these patients.
Fasting period
Eight of the 16 included patients were treated with either a late night snack, MCT, raw or modified corn starch (Glycosade®, Nestlé Health Science) or nocturnal tube feeding in order to decrease the maximum fasting period (Tables 2 and 3). Of the seven patients who used MCT supplementation, six (86%) also used a late night snack, raw or modified corn starch and/or nocturnal tube feeding. Raw or modified corn starch was used by two (25%) of the patients and nocturnal tube feeding was used by one patient.
Dietary intervention during illness
During illnesses the majority of patients (12 out of 16) adjusted their regular diet. Nine (56%) shortened their maximum feeding pause. In addition, three used extra carbohydrates (e.g., dextrin maltose) as supplement and lowered overall fat intake, one added MCT to the diet during illness, and one patient started continuous gastric drip feeding.
Dietary interventions before or during sports
Of the eight patients that practiced sports (Table 1), five used dietary interventions before or during sports (Table 2). Three of them shortened their maximum feeding pause by eating or drinking carbohydrate-rich refreshment before or during sports. One patient added a source of carbohydrates (e.g., dextrin-maltose, Fantomalt®, Nutricia) to his diet and one used a source of MCT before sports.
Height and weight
Table 3 shows height, weight, and body mass index of the included VLCADD patients. The median height of the six male patients (age: ≥18 years) was 184 cm (range: 175–187) and is comparable to the mean of the height of the Dutch male population (180.7 cm). The median height of the female patients (age: ≥18 years) was 166 cm (153–187 cm, n = 5). The mean height in the adult Dutch female population is 167.2 cm (CBS 2016).
There was a large variation in BMI. In the patients younger than 18 years (n = 5; three male, two female) the median Z-score for BMI (TNO 2009) was 1.4 (−0.5 − +2.9). In adult patients (n = 11; six male, five female), eight out of 11 were classified as overweight with a BMI >25.0 kg/m2 of which three patients had obesity with a BMI >30.0 kg/m2. No correlation was found between weight and LC-FAO flux score. Median Z-score for BMI was higher in patients that were on LCT restriction/MCT supplementation compared to those that were not (+2.0 vs +1.2), but this was not significant and no correlation of BMI and LC-FAO flux score could be found in either group (Suppl. Fig. 1a–b). Median Z-score for BMI was lower in patients that practiced sports compared to those that did not (+1.2 vs +2.35), but the difference was not significant and no correlation of BMI and LC-FAO flux score could be found in either group (Suppl. Fig. 1c–d).
Genotype
All patients with homozygous loss-of-function mutations in ACADVL, such as frameshift and splice-site mutations, had an LC-FAO flux score <10% (supplementary Table 1). Five patients were compound heterozygous for one missense and one loss-of-function mutation and all of them had an LC-FAO flux score between 10 and 90%. At diagnosis, most patients had a combination of different missense mutations with unknown effect on the protein. Therefore, genotyping would be insufficient to predict phenotype; hence, the requirement of enzymatic assays, such as VLCAD activity and LC-FAO flux.
Discussion
This study shows that the LC-FAO flux score is a good predictor of clinical outcome in VLCADD patients identified before introduction of this disorder in the NBS panel. Furthermore, a poor clinical outcome could not be prevented by dietary intervention, comprising LCT restriction or MCT supplementation and all patients with a low LC-FAO flux score (<10%) had severe VLCADD-related symptoms despite the use of intensive dietary treatment. VLCADD patients with an intermediate LC-FAO flux score (between 10 and 90%) received different dietary interventions and had variable outcomes which seemed unrelated to their LCT and MCT intake. The one individual with a high LC-FAO score (>90%) had an excellent outcome without any dietary intervention.
The observation that not all VLCADD patients need dietary treatment, including the use of MCT, has been reported previously (Laforet et al 2009; Evans et al 2016; Pena et al 2016). However, in two studies (Evans et al 2016; Pena et al 2016), patients were, in contrast to our cohort, identified by NBS and treated with MCT until the age of 5 years. It may be possible that a severely LCT-restricted diet might have negative clinical consequences, for example on essential fatty acids (Diekman et al 2014). Moreover, there is also evidence that MCT supplementation can be harmful as MCT might be elongated to LCT in certain circumstances (Jones et al 2006; Primassin et al 2010; Tucci et al 2015a, b). Based on our findings we cannot conclude anything on these matters as we did not find any correlation between the amount of MCT and clinical symptoms. In addition, LCT-restriction and MCT-supplementation was only stopped in patients who were asymptomatic. There is one report describing the disastrous effect of accidental LCT loading in a patient who normally used MCT. However, of course, there is a difference between LCT loading and normal intake (Ficicioglu and Hussa 2009).
Prediction of phenotypic severity based only on mutation analysis is often not feasible in VLCADD (Hoffmann et al 2012), which is confirmed by the present study. Biallelic loss-of-function mutations that have a severe effect on protein function, such as splice-site and frameshift mutations, clearly result in a very low LC-FAO flux score and poor outcome. However, this concerns only a small subset of patients. The majority of patients are compound heterozygous for different combinations, including missense mutations. The consequences of such combinations of different mutations on VLCAD enzyme activity and the flux through the pathway cannot be predicted, and this can only be assessed by studies in cultured skin fibroblasts. We previously showed that the LC-FAO flux score, an established method that has been used for several years (Manning et al 1990; Olpin et al 1997, 1999), appears to be the best parameter in predicting the clinical severity (Diekman et al 2015). Hence, we suggest that the LC-FAO flux score in fibroblasts can be used as a method for selecting the optimal therapeutic strategy. In patients with an LC-FAO flux score >90%, the VLCADD can probably be best considered as a ‘risk factor’, which will only lead to clinical symptoms after significant provocation, such as prolonged fasting during an infection. In our opinion there is no need for these patients to use a continuous dietary treatment. Since patients with an LC-FAO flux score <10% have severe clinical symptoms even despite a strict dietary treatment, there is a need for alternative treatment options for these patients. However, we cannot advise to refrain from dietary intervention in this group. Indeed, anecdotal evidence suggests that stopping the diet in patients with a severe phenotype may have deleterious clinical consequences (Ficicioglu and Hussa 2009).
In patients with an LC-FAO flux score in fibroblasts between 10 and 90%, a dietary strategy, based on the clinical course of the disease is probably the best option. It is clear that the ordinal inclusion of hypoglycemia in the CSS obscures the correlation between clinical severity and LC-FAO flux score, especially in the group with an LC-FAO flux score between 10 and 90%. Still, CSS correlates better to LC-FAO flux score than myopathy in this group (Suppl. Fig. 2). Due to this disadvantage, we do not yet propose to use the LC-FAO flux score to distinguish within the 10–90% LC-FAO flux score group, but we strongly feel that it is already a useful tool to distinguish between severe and mild patients and avoid stringent dietary measures in individuals with sufficient beta-oxidation.
A limitation of this study is that patients were treated in different centers, which is probably why some patients with an intermediate flux were treated more aggressively than others. In this group, different approaches along with clinical course of the disease have both attributed to the variety in this group.
Based on our findings, we propose a treatment strategy for individuals with VLCADD identified by NBS, which is shown in Figs. 1 and 2. This strategy has been approved by the advisory board for NBS on inborn errors in the Netherlands and is currently implemented within the Dutch NBS program. Since the LC-FAO flux score can only be measured in fibroblasts it will take at least 3 months before these results are known. While waiting for these results, a maximum feeding pause and emergency advice during illness are prescribed (Fig. 1). If a child is clinically ill when the diagnosis is made, fat intake should be stopped immediately until the child is clinically stable. Caution is warranted if this exceeds 24 h as caloric intake should also be adequate to prevent a catabolic state. Clinical symptoms in this stage immediately result in an LCT-restricted/MCT-supplemented diet. If a patient is asymptomatic after diagnosis, but has a significantly elevated creatine kinase or amino transferases (Fig. 1), the patient will receive an LCT-restricted/MCT-supplemented diet, with a total fat restriction (25–30 E%). The initial dietary restrictions can be alleviated when the child is clinically stable for a longer period of time and LC-FAO flux score is sufficient (Fig. 2).
Dutch guideline for infants diagnosed with VLCADD by NBS before LC-FAO flux score is known. ASAT, aspartate aminotransferase; ALAT, alanine aminotransferase; CK, creatine kinase; E%, energy percentage; LC-FAO flux, long chain fatty acid oxidation flux; MCT, medium chain triglycerides; NBS, newborn screening; VLCADD, very long chain acyl-CoA dehydrogenase deficiency
If LCT restriction/MCT supplementation needs to be started, we advise to follow the recommended guidelines for healthy nutrition and keep caloric intake and carbohydrate intake appropriate for age. Regarding BMI control, weight loss should only be done under strict monitoring and dependent on individual needs. There has been a report in literature about monitored weight loss in patients with long-chain fatty acid oxidation disorders (Zweers et al 2012).
If a patient is asymptomatic after diagnosis and has normal lab findings (Fig. 1) the feeding of the baby can continue as normal, including breastfeeding, but there are strict limitations regarding the maximal feeding pause depending on age. During illness, dietary adjustments such as frequent carbohydrate-rich feedings are commenced.
With regard to the future, since VLCADD has been introduced in many newborn screening programs worldwide, more patients will be detected from an early age. Most likely, the majority of patients identified by NBS will have LC-FAO flux scores >10%.
The nationwide implementation of the LC-FAO flux score will allow us to test the validity of the proposed strategy in VLCADD patients diagnosed by NBS and probably define better cut-off values for dietary adjustments in the future.
References
Andresen BS, Olpin S, Poorthuis BJ et al (1999) Clear correlation of genotype with disease phenotype in very-long-chain acyl-CoA dehydrogenase deficiency. Am J Hum Genet 64:479–494
Arnold GL, Van Hove J, Freedenberg D et al (2009) A Delphi clinical practice protocol for the management of very long chain acyl-CoA dehydrogenase deficiency. Mol Genet Metab 96:85–90
Bach AC, Babayan VK (1982) Medium-chain triglycerides: an update. Am J Clin Nutr 36:950–962
Baruteau J, Sachs P, Broue P et al (2013) Clinical and biological features at diagnosis in mitochondrial fatty acid beta-oxidation defects: a French pediatric study of 187 patients. J Inherit Metab Dis 36:795–803
Behrend AM, Harding CO, Shoemaker JD et al (2012) Substrate oxidation and cardiac performance during exercise in disorders of long chain fatty acid oxidation. Mol Genet Metab 105:110–115
Bonnet D, Martin D, Pascale De L et al (1999) Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation 100:2248–2253
CBS (2016) Leefstijl en (preventief) gezondheidsonderzoek; persoonskenmerken. (Lifestyle and (preventive) health survey; personal characteristics. Centraal Bureau voor de Statistiek - Dutch Institute for for Statistics. http://statline.cbs.nl
Diekman EF, Bleeker JC, van Veen MR et al (2014) Essential fatty acid deficiency in very long-chain acyl-CoA dehydrogenase deficient patients. Int J Food Nutr Sci J Food Nutr Sci 1:27–30
Diekman EF, Ferdinandusse S, van der Pol L et al (2015) Fatty acid oxidation flux predicts the clinical severity of VLCAD deficiency. Genet Med 17:989–994
Evans M, Andresen BS, Nation J, Boneh A (2016) VLCAD deficiency: follow-up and outcome of patients diagnosed through newborn screening in Victoria. Mol Genet Metab 118:282–287
Ficicioglu C, Hussa C (2009) Very long-chain acyl-CoA dehydrogenase deficiency: the effects of accidental fat loading in a patient detected through newborn screening. J Inherit Metab Dis 32(Suppl 1):S187–S190
Hoffmann L, Haussmann U, Mueller M, Spiekerkoetter U (2012) VLCAD enzyme activity determinations in newborns identified by screening: a valuable tool for risk assessment. J Inherit Metab Dis 35:269–277
Jones PM, Butt Y, Messmer B, Boriak R, Bennett MJ (2006) Medium-chain fatty acids undergo elongation before beta-oxidation in fibroblasts. Biochem Biophys Res Commun 346:193–197
Laforet P, Acquaviva-Bourdain C, Rigal O et al (2009) Diagnostic assessment and long-term follow-up of 13 patients with very long-chain acyl-coenzyme a dehydrogenase (VLCAD) deficiency. Neuromuscul Disord 19:324–329
Manning NJ, Olpin SE, Pollitt RJ, Webley J (1990) A comparison of [9,10-3H]palmitic and [9,10-3H]myristic acids for the detection of defects of fatty acid oxidation in intact cultured fibroblasts. J Inherit Metab Dis 13:58–68
Olpin SE, Manning NJ, Pollitt RJ, Clarke S (1997) Improved detection of long-chain fatty acid oxidation defects in intact cells using [9,10-3H]oleic acid. J Inherit Metab Dis 20:415–419
Olpin SE, Manning NJ, Pollitt RJ, Bonham JR, Downing M, Clark S (1999) The use of [9,10-3H]myristate, [9,10-3H]palmitate and [9,10-3H]oleate for the detection and diagnosis of medium and long-chain fatty acid oxidation disorders in intact cultured fibroblasts. Adv Exp Med Biol 466:321–325
Pena LD, van Calcar SC, Hansen J et al (2016) Outcomes and genotype-phenotype correlations in 52 individuals with VLCAD deficiency diagnosed by NBS and enrolled in the IBEM-IS database. Mol Genet Metab 118:272–281
Primassin S, Tucci S, Herebian D et al (2010) Pre-exercise medium-chain triglyceride application prevents acylcarnitine accumulation in skeletal muscle from very-long-chain acyl-CoA-dehydrogenase-deficient mice. J Inherit Metab Dis 33:237–246
RIVM (2011) NEVO-tabel (Dutch nutrient content table). RIVM/Voedingscentrum, The Hague
Saudubray JM, Martin D, de Lonlay P et al (1999) Recognition and management of fatty acid oxidation defects: a series of 107 patients. J Inherit Metab Dis 22:488–502
Solis JO, Singh RH (2002) Management of fatty acid oxidation disorders: a survey of current treatment strategies. J Am Diet Assoc 102:1800–1803
Spiekerkoetter U, Lindner M, Santer R et al (2009) Treatment recommendations in long-chain fatty acid oxidation defects: consensus from a workshop. J Inherit Metab Dis 32:498–505
Spiekerkoetter U, Bastin J, Gillingham M, Morris A, Wijburg F, Wilcken B (2010) Current issues regarding treatment of mitochondrial fatty acid oxidation disorders. J Inherit Metab Dis 33:555–561
TNO (2009) Fifth National Growth Study. TNO, Leiden
Tucci S, Behringer S, Spiekerkoetter U (2015a) De novo fatty acid biosynthesis and elongation in very long-chain acyl-CoA dehydrogenase-deficient mice supplemented with odd or even medium-chain fatty acids. FEBS J 282:4242–4253
Tucci S, Flogel U, Spiekerkoetter U (2015b) Sexual dimorphism of lipid metabolism in very long-chain acyl-CoA dehydrogenase deficient (VLCAD−/−) mice in response to medium-chain triglycerides (MCT). Biochim Biophys Acta 1852:1442–1450
Wanders RJ, Vreken P, den Boer ME, Wijburg FA, van Gennip AH, IJLst L (1999) Disorders of mitochondrial fatty acyl-CoA beta-oxidation. J Inherit Metab Dis 22:442–487
Wanders RJ, Ruiter JP, IJLst L, Waterham HR, Houten SM (2010) The enzymology of mitochondrial fatty acid beta-oxidation and its application to follow-up analysis of positive neonatal screening results. J Inherit Metab Dis 33:479–494
Zweers H, Timmer C, Rasmussen E, den Heijer M, de Valk H (2012) Successful weight loss in two adult patients diagnosed with late-onset long-chain fatty acid oxidation defect. JIMD Rep 6:127–129
Acknowledgments
We thank all patients and their families who participated in this study for their time and efforts.
Funding
Grant from Metakids (Dutch funding organization for research in metabolic diseases).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J.C. Bleeker, I.L. Kok, S. Ferdinandusse, M. de Vries, T.G.J. Derks, M.F. Mulder, M. Williams, E.R. Gozalbo, A.M. Bosch, D.T. van den Hurk, M.G. M. de Sain-van der Velden, H.R. Waterham, F.A. Wijburg, and G.Visser declare that they have no conflict of interest.
Additional information
Communicated By: Avihu Boneh
Electronic supplementary material
Suppl. Fig. 1
Z-score for BMI of included VLCADD patients correlated to diet and sports (DOCX 1999 kb)
Suppl. Fig. 2
Interim correlations of hypoglycemia, myopathy, CSS, and LC-FAO flux score of included VLCADD patients with an LC-FAO flux score between 10-90% (DOCX 1211 kb)
Suppl. Table 1
Biochemical and molecular characteristics of included VLCADD patients (DOCX 18 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bleeker, J.C., Kok, I.L., Ferdinandusse, S. et al. Proposal for an individualized dietary strategy in patients with very long-chain acyl-CoA dehydrogenase deficiency. J Inherit Metab Dis (2018). https://doi.org/10.1007/s10545-018-0164-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10545-018-0164-5